
Vegetation mosaic of evergreen gallery riverine forest and dry woodlands of deciduous species in higher elevations in seasonally wet, tropical Ethiopia (Photo: K. Müller-Hohenstein)
18.1 Introduction
The previous chapter showed how plants and plant communities develop over time and focused on short- and long-term temporal dynamics. These temporal dynamics lead to varying distributional patterns of species and communities. It is the task of spatial ecology to recognise such distribution patterns, to describe them, and to mechanistically explain their formation. Recognition and understanding of both the temporal and spatial dynamics of plant communities are indispensable for a thorough comprehension of biotic interactions within ecosystems, which is the topic of discussion in this chapter. The current chapter deals with the ecological basis of plant distribution, followed by a discussion of the relationships between species and area, with a particular emphasis on island biogeography.
18.2 Plant Dispersal

Plants have many different methods of effective dispersal. a Aeloropus littoralis is a salt-tolerant coastal grass which spreads by vegetative runners (blastochory) as in south-western Arabia. b Self-dispersal (autochory) in Trifolium stellatum takes a special form (herpochory) with awns on the fruit having hygroscopic characteristics which enables the seed to move over short distances. c A special form of secondary animal dispersal (zoochory) is provided by coprochory. Dung beetles (many types of scarabs) collect the droppings of animals (e.g. cattle) and form it into balls. The dung contains seeds that are then buried just below the soil surface, where they may have a chance to germinate (Photos: K. Müller-Hohenstein)
Successful plant dispersal is typically improved when propagules possess certain morphological traits that improve their likelihood of finding an ideal site on which to become established. These traits can take advantage of external vectors to facilitate transport, which could include wind, water or even animals.
18.2.1 Traits and Vectors
Major dispersal strategies of fruits and seeds (after Howe and Smallwood 1982)
|
Dispersal agent |
General adaptation |
Modification |
Derivation |
Comment |
|---|---|---|---|---|
|
Animal |
Chemical attractant |
Aril, pericarp, pulp |
Seed coat or floral part |
Vertebrate dispersal |
|
Fleshy nutrient |
Elaiosome |
Seed integument |
Ant dispersal |
|
|
Clinging structures |
Hooks, viscous material |
Usually floral parts |
Sticks to fur or feathers |
|
|
Mimesis |
Coloured seed coat |
Seed coat |
Eaten by birds |
|
|
Wind |
Size reduction |
Dustlike seeds |
Seeds |
Up to millions/plant |
|
High surface/volume ratio |
Wings, plumes, balloons |
Seed coat or fruit |
Balloons uncommon |
|
|
Tumbleweeds |
Shoot breaks loose |
Entire shoot |
Seeds scattered |
|
|
Water |
Resistance to sinking |
Hairs or slime |
Seed coat |
Submerged transport |
|
Uses surface tension |
Small size, unwettable |
Seed coat |
Float until wetted |
|
|
Low specific gravity |
Air spaces, cork, oil |
Seed or fruits |
Floats long distances |
|
|
Self dispersal |
Explosive fruits |
Varied |
Fruits |
Secondary transport common |
|
Creeping diasporas |
Hydroscopic bristles |
Fruits |
Occurs with varying humidity |
Relationship between propagules and dispersal by vertebrates (after Howe and Westley 1986, with additions)
|
Animal/animal group |
Propagule colour |
Propagule smell |
Propagule form |
Use to animals |
|---|---|---|---|---|
|
Mammals in herds |
Brown |
Little smell |
Thick husked nuts, do not burst open |
Seeds |
|
Birds in flocks |
Green, brown |
Without |
Seeds without wings and small nuts |
Seeds |
|
Frugivor mammals in trees |
Yellow, green, white, orange, brown |
Aromatic |
Seeds often with arils, whole fruits, burst open |
Arils, pulp rich in proteins and sugars |
|
Bats |
Green, white, light yellow |
Aromatic, musty |
Diverse, often pendent fruit |
Lipid- and starch-rich fruits |
|
Ground living and frugivorous mammals |
Green, brown |
Without |
Hard, over 50 mm long fruits, do not burst open |
Lipid-rich fruits |
|
Frugivorous birds (obligate) |
Black, blue, red, green |
Without |
Big seeds with arils, whole seeds often burst open |
Lipid- and protein-rich fruit flesh |
|
Frugivorous birds (facultative) |
Black, blue, red, white |
Without |
Small seeds with arils, berries and stone fruits |
Mostly carbohydrate-rich fruit |
|
Furry or feathery |
Insignificant |
Without |
Sticky and barbed hooks |
None |
Dispersal vectors may change over time depending on environmental conditions. Species that can use multiple dispersal vectors are considered “polychor”. Many plants do not use only one vector but possess different morphological adaptations to exploit several vectors. The ability to use several strategies improves a plant’s chances of finding an ideal site for establishment. In special cases, however, a “secondary” dispersal strategy could prove more important. For example, an African elephant eats its favourite fruit, containing seeds, which are passed later in the day, 20 km from where they were eaten (primary dispersal). The surviving seeds are then further dispersed by dung beetles, which transport the seeds over short distances before burying them (secondary dispersal) (Fig. 18.1c) (Engel 2000). The importance of “secondary” dispersal is often underestimated. The example of dung beetles shows that the most favourable microsites are often only found in the secondary transport phase. Secondary transport may also lead to a wider range of dispersal.
- 1.
Propagation by the plant itself (autochory)
- 2.
Propagation by other vectors (allochory)
18.2.1.1 Autochory
Over time, plants have evolved various mechanisms to ensure successful dispersal. In its simplest form, autochory, propagules are dispersed via gravity. Typically, the highest proportion of seeds is found closest to the parent tree, which is also known as the seed shadow. On rare occasions (i.e. steep slope or following a disturbance), dispersal can occur over longer distances. Such plants have no special traits for dispersal.
Propagules can also reach suitable growing sites by growth processes, for example, via scions (vegetative shoots) or flower stalks (pedicels, e.g. Cymbalaria muralis). Propagules are even able to move by themselves over short distances, for example, by twisting awns due to changes in humidity (Fig. 18.1b). In addition, some plants have even evolved mechanisms to project propagules by a single impulse or shot, which helps ensure further distances travelled from the parent plant. The propagule ejections can be triggered by a series of different vectors: animals, wind or even a simple raindrop (e.g. Impatiens noli-tangere). In some species, these triggers are not required, where certain changes in abiotic factors must occur for projection of the propagule to occur. For example, differences in turgor pressure in a plant (e.g. Ecballium elaterium) or changes in moisture exchange through the seed coat (e.g. Bauhinia purpurea) can trigger this phenomenon. Seed dispersal through this mechanism can easily cover several metres in some cases.
18.2.1.2 Allochory
Wind is an important abiotic dispersal vector for allochoric seed transport (anemochory). Even when seeds reach the ground or soil substrate, they can continue to be blown along the surface. Plant propagules have been able to adapt morphologically to ground dispersal, especially in dry arid regions of the world where there are rarely impediments or barriers to such transport. More importantly, however, is transport by air currents. Small, light propagules are particularly benefactors of this mode of dispersal as they can easily be dispersed multiple times under the right gusts of wind, for example, seeds of orchids or spores of cryptograms. Some other interesting wind-dispersed adaptations include balloon fliers (e.g. Astragalus spinosus) that possess special morphological adaptations, as do seeds or fruits with parachutes (e.g. Taraxacum officinale). A number of tree species (e.g. Acer and Fraxinus spp.) have adapted special wings (or samaras) to help keep propagules in the air and increase dispersal distances. Wind also triggers seed transport in plants that scatter seeds (e.g. Papaver spp.).
Hydrochory involves the transportation of propagules either in or with the help of water as a dispersal vector. Some propagules are able to float because of special tissues or large intercellular spaces that have a low specific weight and that typically possess a hydrophobic seed coat, which makes it difficult for the seed to absorb water (e.g. Nymphea species). Coconuts and certain seeds and seedlings of some mangrove species are excellent examples of this adaptation, where they are able to stay in salt water for extended periods of time with little or no effect on seed viability. A seed that falls into a moving body of water (i.e. river, lake or ocean) can almost ensure successful long-distance dispersal; however, a body of water is not always required. In drier, more arid areas, seeds can travel substantial distances from their parent plant with the help of a few raindrops (e.g. Anastatica hierochuntica in dry regions). There are disadvantages to this mode of dispersal, however, as floating for prolonged periods of time can cause premature germination with a lack of an anchoring substrate. Plants usually prepare their offspring for this encounter by certain dormancy cues, where a seed must meet specific environmental conditions (typically moisture and temperature) prior to breaking dormancy and allowing germination to ensue.
The most important and ecologically most complex form of allochoric dispersal that exists is zoochory. Zoochory involves the use of animals as dispersal vectors for plants. Plants have developed different mechanisms and traits to entice or attract animals to disperse their seed, where, interestingly, animal foraging and behaviour can determine patterns of seed dispersal. Close interrelations between some plant and animal species point towards a long co-evolutionary development. This applies particularly to endozoochory, where propagules are transported internally by the animal. To do so successfully, seeds should have a relatively hard shell that ensures they are resistant to conditions within the animal’s digestive system (i.e. high acidity). After excretion, seeds are usually provided with good starting conditions for germination in the nutrient-rich excrement. Although propagules can be taken up randomly, very close links between birds, bats and herbivores with plant species through the use of fruit can entice attraction and increase the likelihood of seed dispersal. These are especially evident in the humid tropical regions of the world (Table 18.2). Zoochory does have some disadvantages as a dispersal medium; foraging animals can hoard or cache seeds (i.e. squirrels) in a safe site, which could reduce the likelihood of future germination (e.g. seeds of Pinus cembra collected by Nucifraga caryocatactes), but some of them still have a chance to survive because not all hiding places are found again and some seeds are also lost during transport.
Birds (ornithochory), bats, ants (myrmecochory) and many larger animals are especially attracted to the fruit of plants. Plants invest in nutrient-rich fruits and have evolved to attract dispersal vectors by the use of bright vibrant colours and strong odours. Ants may carry seeds possessing lipid-rich elaiosomes (e.g. Corydalis cava), but the seeds remain untouched. Birds are especially attracted by the colouring of fruits and are even able to distinguish ripe seeds from unripe ones. Sometimes propagules are eaten or damaged. These are often starch-rich and can provide nourishment for an animal. In some instances a monkey, for example, will ingest the fruit pulp and then spit out the seed.
Epizoochory is a form of passive dispersal by animals, where the seed possesses mechanisms to cling to the hair or fur of the animal. Typical mechanisms include glue-like excretions, glandular hair, barbs with awns and other outgrowths formed from the pericarp that can attach to the animal. By doing so, the seed greatly improves its dispersal distance; however, unlike in earlier cases, the animal is not rewarded for its transportation through nutrient supplementation. In endozoochory, close interaction exists between plant and animal, where dispersal is regulated, sometimes even targeted. As a result, epizoochory is mainly random.
Two additional dispersal mechanisms exist that do not really fit well with the previously mentioned groups. The first is atelochory (also called achory), where dispersal does not occur, and in fact it is prevented. The consequence of this evolutionary development is that reproduction takes place at the site where the mother plant grows, which is favourable to the species. This lack of dispersal allows the offspring to take advantage of similar conditions its parents have. Arachis hypogaea or Trifolium subterraneum provide examples of such a strategy, where, following pollination, the pedicel and ovary penetrate into the soil and become established.
The final mode of dispersal for propagules involves humans (anthropochory), which with the onset of the Anthropocene has played an increasingly important role in the recent history of plant dispersal. In this particular form, any dispersal distances can be achieved, and essentially, no geographical or ecological barriers exist. Anthropochory dispersal occurs when humans are looking to move certain species of plants to specific areas for either food or ornamental purposes. If dispersal occurs unintentionally along with other propagules (e.g. weeds disperse along with crops of cereal), their distribution and the pattern of the species concerned typically almost always occur randomly. Both forms are closely linked to the problems with neophytes (Sect. 17.3).
18.2.2 Effectiveness of Dispersal Mechanisms
Dispersal can be limited by physical barriers, such as large differences in altitude of mountainous regions, oceans and large arid regions. The absence of certain environmental conditions or requirements (i.e. temperature, water, light, soil nutrients, symbiotic patterns) may significantly limit successful establishment following dispersal. Keep in mind that these barriers or requirements can be manipulated in plants favoured by the human vector, where people can improve growing conditions by removing competition, improving soil conditions through fertilisation or aeration.
A clear understanding of how far propagules can disperse is especially important in the field of ecosystem restoration, which could provide key information about species’ ability to persist in fragmented habitats. As this fragmentation is quite common in human-altered landscapes, better knowledge about successful seed dispersal is indispensable. This is especially true for ballistic and other autochoric species, including ant- and bat-dispersed vectors, where dispersal distances travel is rarely beyond a few metres. In contrast, many vertebrates, wind and water are able to disperse propagules for distances exceeding 10 km. In some of the best-case dispersal distance scenarios, orchid seeds and spores from ferns have been known to travel distances of several hundred kilometres.

Dispersal mechanisms can change during succession. Relative frequency of four dispersal mechanisms in phanerogamic flora of fallow fields close to Montpellier (France) at different times after their abandonment (after Lepart and Escarre 1983)
The dispersal of propagules is usually deemed successful if they reach a site that is favourable for their germination and establishment and ensures growth till flowering and seed production (safe site). Following germination and establishment it is extremely important to the long-term survival of the species that it exploit its new site, which is at some distance from the parent plant, contributing to the expansion of the species range or area. An expanding range or shift provides a few benefits, especially if it occurs at fast enough rates. A fast shift can allow a plant species to escape certain pathogens and enhance the population’s chances of survival. To a large degree this depends on the available mechanisms for dispersal and their effectiveness regarding a targeted (suitable site for growth) and far-reaching (gain space) transport of propagules. However, such substrate “targets” are typically not achieved through a single form of dispersal. To ensure successful colonisation, a series of different dispersal vectors and their associated interactions can be used to improve seed dispersal (heterospory). Multiple dispersal mechanisms can supplement each other to ensure successful dispersal. Under unfavourable external conditions (i.e. a short vegetation period in high mountains or long dry periods), reproductive dispersal is combined with vegetative propagation. For example, the alpine species Polygonum viviparum produces both seeds and bulbils on the same stalk, with the bulbil fraction increasing with altitude.
These particular advantages associated with specific regions typically occur when the species adapts to the local conditions over time (local adaptation). For instance, wind-dispersed propagules in arid regions are often transported over long distances because only minimal obstacles occur over land. Another example of plant-dispersal local adaption is the production of flashy or tasty fruit-bearing plants, which increase the likelihood of attracting animal dispersal vectors. Essentially, different strategies have evolved in which successful arrival at safe sites and spatial expansion provide a niche balance. Studies on seed dispersal in forests have shown that many trees in the humid tropics with rainy and dry seasons have created morphologically adapted seeds that facilitate transportation by animals. In temperate forests this also applies to shrubs, but here seeds from trees are predominantly transported by wind. This change in the form of dispersal is explained by regular winds occurring in temperate latitudes. Also in dry areas, where almost no obstacles impede air currents, wind dispersal dominates. All this is to say that there is no concrete evidence for certain dispersal vectors being exclusively used or associated with specific biomes (Tables 18.1 and 18.2).
Several hypotheses and models have been developed to help explain the link between certain plant species and their vectors. The low investment model is associated with plants’ investing little per propagule produced, resulting in a large number of small seeds. This model favours dispersal vectors associated with long-distance dispersal (wind-dispersed, attached to animals). Because little is invested in each seed, the recruitment rate is quiet low; however, this can be offset by volume. This strategy is typically used by early successional species. The second model, the high investment model, is essentially the opposite, where plants produce very few, large seeds. The large seed size provides ample amounts of energy and nutrients to ensure good rates of germination, assuming it attracts the appropriate dispersing agent (bird or bat) and falls upon a favourable substrate. The seeds in this strategy are only distributed over short distances, but they usually find relatively safe conditions for germination. In animal-dispersed species, both strategies are used: seeds externally attached to animals (with low investment) and those transported in the gut (with high investment). The directed dispersal hypothesis first proposed by Howe and Smallwood (1982), regarding targeted, relatively safe dispersal, also aligns with the high investment model. This contrasts with the colonisation hypothesis, where opportunists exploit opportunities for rapid dispersal over large areas. The premise for the escape hypothesis is that the chances for establishment and germination depend on the low density of conspecifics, so the mother plant cannot be nearby (e.g. Janzen-Connell effect) (Sect. 19.3).
Factors affecting efficiency of dispersal (after Schupp 1993)
|
I. |
Quantity of seed dispersal |
|
A. |
Number of visitors |
|
1. Density of dispersal agent |
|
|
2. Type of nutrition |
|
|
3. Reliability of the visit |
|
|
B. |
Number of seeds dispersed per visit |
|
1. Number of seeds touched per visit |
|
|
2. Probability of dispersal of a seed touched |
|
|
II. |
Quality of seed dispersal |
|
A. |
Quality of treatment |
|
1. Seeds are transported intact or broken |
|
|
2. Change in germination rate |
|
|
B. |
Quality of seed deposition |
|
1. Transport type |
|
|
(a) Targeted choice of habitat |
|
|
(b) Targeted transport |
|
|
2. Deposition type |
|
|
(a) Proportion of deposited seeds |
|
|
(b) Mixing of different seeds |
18.2.3 Propagule Bank and Seedling Establishment
Seed dispersal is deemed successful only if the transported propagules germinate at a potential site or are incorporated into the soil (in which case it will become established at a later time). The stock of all dispersal units in the soil is called the propagule or seed bank, also a seed pool. Seeds may remain viable for several years, in some cases up to a few centuries, until more favourable conditions for development occur. Seeds from Medicago lupulina have been found to be viable in the soil after 26 years, significantly lower than the seed of Spergula arvensis, which has been shown to maintain viability up to 1700 years (Urbanska 1992). Many propagules reduce metabolic activity before germination. Germination-inhibiting chemical substances may prevent further development. Sometimes temperatures must fall below a certain minimum value (vernalisation) before the seedling can develop. Such periods are called dormancy and may be determined genetically or by external conditions. Many seeds are protected by a thick pericarp, which can be impermeable to water. Dormancy acts as an environmental checklist for the seed, where until all environmental condition (typically light and temperature) cues are checked off, the seed will remain in the soil until conditions are met. The amount of time a seed will remain viable in a seed bank will vary from species to species.
Baskin and Baskin (2001) identified the differences between dormancy types; the first, endogenous dormancy, is where the properties associated with the seed embryo inhibit germination (e.g. physiological inhibiting mechanisms or an undeveloped embryo), whereas exogenous dormancy is described as where the properties of the endosperm or any other tissues of the seed or fruit inhibit germination (e.g. physical, chemical or mechanical constraints on germination and embryo growth).
The period of dormancy is different for different types of propagules. In tropical rainforests, seeds of shade-tolerant trees will tend to germinate immediately if they are found within a suitable growing site. Seeds of shade-tolerant trees often remain dormant until favourable conditions emerge, which will then allow for germination to occur, for example, after a fallen tree opens a gap in the canopy. In dry regions with very variable wet periods, species differ considerably in dormancy, even within individuals of the same species. In general, there is a clear favourability for seeds to be non-dormant in wet tropical rain forests (60% of species are non-dormant), mirroring an environmental gradient of decreasing precipitation and increasing seasonality or uncertainty of rainfall events. Generally, it may be assumed that the dormancy of propagules serves to tune germination to growth conditions that will provide a suitability bridge for a cold winter or a dry season.
A seed bank is usually located in the uppermost 10 cm of soil. Seed banks are made up of two types: temporary and long-term. Temporary propagule banks consist of the accumulation of seeds that will germinate in the short-term (next year). Long-term propagule banks are particularly important for the regeneration of plant communities. Propagules in temporary banks have only limited ability to germinate—as for tropical shade trees—and the regeneration of forests after a severe disturbance is unlikely. This also applies to rainforests in southern Chile outside the tropics. A few years after clearing of the almost natural stands and reforestations of the area with Pinus radiata, the local seed banks contained hardly any propagules of indigenous species (Scherer and Deil 1997). This underlines the importance of seed banks for the protection of species and biotopes.
The phase of seedling establishment is particularly sensitive in the life cycle of plants. Pathogens, herbivores and competitors, but also climatic abnormalities, may lead to large losses. Some seedlings require the protection of neighbouring species (e.g. Arabis hirsuta, Primula viridis), but for others germination is reduced by neighbouring species (Plantago lanceolata, Sanguisorba minor). A third group of species was able to germinate under all experimental conditions (e.g. Medicago lupulina). Large, time-dependent fluctuations have also been observed. There are close relations between rates of germination and interannual fluctuations of climatic conditions, particularly of temperatures. Most species germinate more successfully at higher temperatures, while others germinate better at lower temperatures (Espigares and Peco 1993). In some species of Acer, seed germination can commence only if it experiences a certain number of cool, moist days (known as stratification) near the freezing point to break dormancy and allow for germination to ensue (Solarik et al. 2016).
While the molecular and biochemical bases for dormancy and germination are well known for certain model species under controlled environmental conditions, we still lack a clear understanding of some of the fundamental processes under natural conditions that inhibit or promote seed germination and establishment. The successful establishment of a plant population is only secured by a permanent input of propagules, even if edaphic and climatic conditions at the growing site are suitable, pollinators (when necessary) are present, and the species is able to successfully compete with co-occurring individuals and protect itself against pathogens.
18.2.4 Distribution Patterns
As a result of different dispersal mechanisms, plant species develop distinct spatial patterns of distribution. These patterns are not permanently fixed in space because they depend largely on abiotic and biotic factors, which continuously fluctuate over time (daily, weekly, monthly, seasonally and yearly). Among these factors especially those related to climate, soil and mechanical impacts (flooding, thunderstorms) and—in cultural landscapes—agrochemical and agro-technical influences are important. Accurately identifying the propagule distribution pattern will depend on the spatial scale, where variability from the local-scale small patches to large-scale landscapes must be considered. A several-square-kilometre area with small forest islands, grasslands and bogs will show different patterns when compared with dispersal at finer scales (cm2, m2) in the same area, which typically will yield much different results. Today, a species’ distribution is typically assessed by the use of a grid system in analysis. Although results will depend on the size of the grid chosen for analysis, the spatial scale (e.g. “patch”, “local” or “landscape”) becomes essential when considering dispersal patterns.
Distribution patterns will typically fall under three main types: clumped, regular and random distributions. The clumped distribution is the most common type in natural vegetation, mainly because of a close relationship between abiotic and biotic resources associated with plant establishment and growth. As these resources often present a mosaic structure, species and plant communities often become clumped. The introductory photo of Part IV shows an example of clumped distribution in a tropical high-mountain belt with abiotic site factors changing on a large scale. On a smaller scale, spatially aggregated clumped distribution can be the result of a restricted seed shadow, where seed is either dispersed or animals fail to travel far before excreting or dropping the seed. Fangliang et al. (1997) found in the highly diverse tropical rainforests of Malaysia a 4:1 relation of clumped to random distributed tree species, which suggests a strong influence of animal-dispersed seeds in these systems.
A regular distribution occurs when the propagule is dispersed evenly over an area, where a comparable amount of seeds are found at long and short distances from the parent tree. This distribution pattern is rarely found in nature. The regular distribution can occur when the environmental conditions across the dispersal distance change in a regular way: the zonation of different plant community changes with a significant change in the landscape. Vegetation along a riverside is an example. The mechanical force of the water during the rainy season and the different water storage capacities of the sediments on the low fluvial terraces with different grain sizes can result in the formation of a regular sequence of different species and communities (Fig. 17.25). Regular patterns are also sometimes observed in tropical dry woodlands (“leopard skin”) if the distance between plant individuals is the result of competition for water, the limiting resource. The introductory photo of Chap. 18 shows an example of regular vegetation patterns at the landscape scale, with an evergreen gallery riverine forest and a seasonally moist dry woodland. Most examples of regular patterns found in nature are actually artificial, for example, in fruit tree plantations or even crop cultivation (introductory photo in Chap. 17).
Although rare, regular spatial patterns are typically determined by animals and wind vectors, they are most important for random distributions. One precondition is that the plant species concerned must be generalists, that is, they have a wide resource-based spatial niche (area where species-specific requirements are met), and the spatial distribution of the environmental resources is rather homogeneous. Abiotic and biotic factors are thus spaced in an unpredictable way, as are the individuals of plant species.
18.3 Vegetation Geography
One consequence of short-range dispersal is that the site where propagules fall to the ground is already occupied by conspecific plants, often offspring of the same mother plant. Hence, growing seedlings will typically face intraspecific competition because a favourable abiotic environment is likely. In contrast, for propagules transported by long-distance dispersal, there is typically a higher chance of facing unfavourable abiotic and biotic factors. Although the likelihood of establishment at less favourable sites is lower, a successful establishment can still lead to spatial expansion. The result is an increased species range or area, which is a geographically defined region where individuals of a species can be found.
The science of spatial distribution of plants (chorology) aims (1) to recognise the various types and characteristic patterns of distribution, which provide the ability to map and to describe species organisation, and (2) to explain the development of patterns of distribution. A comparative assessment of geographical areas leads to the characterisation of floristic elements and areal types or geo-elements.
18.3.1 Characterisation and Interpretation of Areas
There are various ways to map areas. Frequently, when considering species distributions, only borders are drawn without providing any additional information concerning the distribution and abundance of species within the area. This can be avoided by creating dot maps, which can provide species locations. The most popular way to represent species distributions is through the use of species range maps. However, these maps often show only a portion of the entire range and rarely contain any information about species abundance. Such maps also show the climatic differentiation of areas often better than those based on meteorological data. Regardless of their shortcomings, these maps are indispensable for applied tasks, for example, nature protection.
The species range or area is essentially the spatial distribution in which a biological taxon is found, that is, where the environment is typically favourable for that species. The range limits are typically controlled by a number of historical and ecological factors, for example, climate, interspecific (other species) and intraspecific (same species) competition, site quality, food resources, water and landscape. Isolating the contribution of each of these factors individually is extremely difficult, especially since they can be co-dependent on each other. One attempt at determining range limits has been done through the use of isotherms (average temperatures); however, plants rarely react to average conditions. For example, the eastern species range of beech (Fagus sylvatica) is constrained by the lack of precipitation; however, another constraint is the severe cold winter (> −30 °C), which is known to cause extensive damage to bud development. At the species’ southern range limit, the opposite occurs, where a combination of a lack of summer precipitation and high temperatures causes significant drought events. Finally, beech’s northern limit has been determined by a combination of prolonged winters and late-season frost events. However, it has been speculated that beech reached its northern limit in the north-west of the British Isles after the last glaciation. These observations have relied on the current distribution patterns of adult trees while ignoring environmental constraints on seed germination and seedling establishment, key processes in determining any long-term presence of a species. This will be of special importance in studies about climate-change-driven dynamics as species attempt to maintain their climatic niches by expanding their ranges in altitude and latitude. As decisive for alpine plant communities and their upper distribution boundary is summer frost resistance (Taschler and Neuner 2004).

Limit of the distribution of olive trees (Olea europaea) in the Mediterranean area and the most important climatic factors determining it (after Müller-Hohenstein 1981)
It should be mentioned that species range limits are dynamic, under constant flux, and can change quite readily owing to climatic factors influencing the biological system. The postglacial retreat of ice caused by climate warming led to significant species range shifts, which made it possible to make inferences on the rates of possible migration. For some important Central European tree species a range expansion of approximately 100 m/year was calculated, a considerable rate when one considers that trees are sessile organisms that typically require decades to reach reproductive maturity. Furthermore, quiet often many tree species will produce ample seed crops only once every several years (masting).

Possible means by which plants exploit space over a period of time. Time axis vertical, space axis horizontal, cut surfaces show the current situation. a Area expansion; b death of populations and shrinkage to disjunct areas; c as in b with development of relict palaeoendemic areas; d allopatric differentiation of three vicarious related groups; e pseudovicarious species, living in ecologically or geographically similar conditions; f distribution of closely related species with a centre of diversity (Z), relict endemics (R) and neoendemic species (A). There are no fixed relationships between the age of the species, diversity of the group or size of the area (from Strasburger and Sitte 1998)
Species ranges are also characterised by their (1) size, (2) form and (3) geographical location. The differentiation between cosmopolitan and endemic species is based on the size of distribution areas. Cosmopolitan species typically occur over large areas, commonly extending over continents and different climatic zones. These species have extremely effective dispersal mechanisms, are highly competitive and can be categorised as phylogenetically old. Some common examples of cosmopolitan species include bracken (Pteridium aquilinum) and annual meadow grass (Poa annua). Cosmopolitans are differentiated from species that are generalists in terms of their site requirements (e.g. Pinus sylvestris).
Proportion of endemics in the flora of different islands and island groups (after Frey and Lösch 1998)
|
Island/island group |
Endemics in % |
Distance to the nearest mainland (in km) |
|---|---|---|
|
Fernando Po |
12.0 |
100 |
|
Canary Islands |
53.5 |
170 |
|
Sao Tomé |
19.4 |
250 |
|
Cape Verde Islands |
15.0 |
500 |
|
Juan Fernandez |
66.7 |
750 |
|
Madeira |
10.5 |
970 |
|
Galapagos |
40.9 |
1120 |
|
Azoren |
36.0 |
1460 |
|
St. Helena |
88.9 |
1920 |
|
Hawaii |
94.4 |
4400 |
|
Marquesas |
52.3 |
6000 |
Species areas are also differentiated by their form, particularly whether they are closed areas, that is, the species is established in a single, clearly delimited space, or whether the area consists of several partial areas and is thus disjunct. In closed areas, gaps between individual growing sites are so small that they may be bridged easily and quickly by the transport of propagules or pollen. In disjunct areas, this is no longer possible. It may be assumed that polyphyletic origin of the same species does not occur and therefore other explanations for the genesis of disjunct areas must be found. One explanation might be an extremely rare distribution event, for example, by migrating birds or through some anthropogenic influence. In many cases, however, it is known that present disjunct areas were once closed and were separated by tectonic events (e.g. continental drift), the formation of mountains or climatic changes (e.g. change in cold and warm periods). A good example of the latter are arcto-alpine species, which were widely distributed in Europe during the Pleistocene, growing in the lowland tundra habitats between the Scandinavian and alpine glaciers. Today, under a warmer period, they are restricted to high alpine and arctic habitats, as well as to isolated patches in lower mountain ranges in Central Europe (e.g. Dryas octopetala).

-
Limestone sites are usually drier than siliceous sites, because the water from precipitation seeps rapidly into deep soil layers.
-
Limestone sites are warmer than siliceous sites because of the lower soil water content.
-
Siliceous: deficient in Mo and alkaline cations but with an excess of Al.
-
On limestone sites litter decomposition leads to mulch (mull) as the dominating form of humus, while siliceous sites are characterised by raw humus or moder.
-
N mineralisation on limestone sites leads to nitrate as the dominating N form, whereas on siliceous sites it is ammonium.

Relationship between calcareous and siliceous vegetation of two alpine plant ecosystems. Matgrass communities (Nardus stricta) occur on siliceous sites, and bluegrass meadows (Sesleria coerulea) occupy calcareous sites. The number of species unable to invade the other’s root space, either because of root competition or other abiotic factors, is shown (after Gigon 1987)
If phylogenetically related species are distributed closely together, it may be concluded that they have developed within the same region and that the area is a centre of diversity (genetic centre, central zone of related groups), or at least a maintenance centre of this genus. Plant breeders aim to find such centres for economically important plants because they hope to find important gene reserves.
18.3.2 Area Types-Floristic Elements-Plant Kingdoms
Areas of similar basic structures, size and geographical positions are categorised into area types. Various species do not occupy absolutely identical areas, but all species of the same area type are considered to be geo-elements if spatial aspects are considered more and floristic elements if floristic aspects are considered. Nevertheless, these terms may be regarded as synonyms.

Classification of geoelements (similar area types) of Central Europe. atl atlantic, arct arctic, bor boreal, eu European, m central, med mediterranean, pont pontic, russ russian, saharo saharian, sindic indian, tur turanic, e east, s south, w west (after Kreeb 1983)
-
plan (planar belt of lowlands).
-
coll (colline belt).
-
mont (montane belt).
-
subalp (subalpine belt to the timberline).
-
alp (alpine belt).
-
niv (nival belt).
-
atl (atlantic).
-
cen (central European).
-
ssib (southern Siberian).
Examples of types of central European geoelements and neighbouring geoelements
|
Central European (eu-mi): |
Fagus sylvatica, Quercus petraea, Hedera helix |
|
Sub-arctic (subarct): |
Betula nana, Salix herbacea, Rubus chamaemorus |
|
Boreal (bor); |
Picea abies, Larix decidua, Ledum palustre |
|
Atlantic (atl): |
Erica tetralix, E. cinerea Sarothamnus scoparius, Ilex aquifolium |
|
Central Russian (mi-ru): |
Carpinus betulus, Quercus robur, Alnus glutinosa, Melampyrum nemorosum |
|
Sub Mediterranean (submed): |
Acer monspessulanum, Quercus pubescens, Sorbus torminalis, Bromus erectus |
|
Mediterranean (med): |
Quercus rotundifolia, Arbutus unedo, A. andrachne |
|
Pontic: (pont): |
Adonis vernalis, Anemone sylvestris, Stipa pennata, S. capillata |
|
South-Siberian (ssib): |
Daphne mezereum, Betula verrucosa, Astragalus danicus |
|
Arctic-alpine (arct-alp): |
Loiseleuria procumbens, Poa alpina, Arctostaphylos alpinus |

The position of plant kingdoms or climatic zones on the Arabian Peninsula. a Position of the boundaries between the Holarctic and the Palaeotropic plant kingdoms according to plant geographers Diels (1908, dotted line), Al Hubaishi and Müller-Hohenstein (1984, continuous line) and Kürschner (1986, broken line). b Boundaries between the subtropics and tropics on the Arabian Peninsula according to climatologists Troll and Paffen (1964, dotted line), von Wissmann (1964, continuous line), Blüthgen (1964, broken line) (after Müller-Hohenstein 1988)

Plant kingdoms and floral regions (after Richter 1997)
The Holarctic is the largest plant kingdom with distribution centres of many plant families (Apiaceae, Betulaceae, Brassicaceae, Caryophyllaceae, Fagaceae, Primulaceae, Ranunculaceae, Rosaceae, Salicaceae). Current differences, for example, between holarctic regions in North America and Eurasia, are caused by recent geological events (ice ages). However, in more recent geological time periods, no insurmountable barriers have arisen.
This does not apply to the southern, tropical regions. The separation of the African continent from South America led to the subdivision of two tropical plant kingdoms; this subdivision is justified, despite existing parallels (pantropical species and families, e.g. Annonaceae). Particularly characteristic families in the Palaeotropics, including the African continent and the South-East Asian archipelagos, are Combretaceae, Dipterocarpaceae, Euphorbiaceae, Moraceae (with over 1000 species of the genus Ficus), Nepenthaceae, Pandanaceae and Zingiberaceae. For the Neotropics, including most parts of Central and South America, species of Araceae, Bromeliaceae, Cactaceae and Solanaceae are particularly characteristic. Tropaeolaceae are entirely limited to this plant kingdom.
The Capensis in the south of the African continent is the smallest plant kingdom, but it is a particularly autonomous realm, where some families have developed into many species. This applies particularly to Ericaceae and Mesembryanthemaceae. In these two families relations to the Holarctic and Antarctic become obvious. The Bruniaceae is an endemic family of the cape, and representatives of the Proteaceae and Restionaceae are dominant.
The Southern Hemisphere plant kingdom of Australis comprises only the Australian continent and Tasmania; at the level of genera and species it is rich in endemic species. Eucalyptus species (Myrtaceae) are particularly important and are now distributed worldwide. The neighbouring New Zealand belongs in part to the Palaeotropics, but in the south partly to the Southern Hemisphere, the Antarctic, which has its largest area almost completely inhabited by plants. However, in the southern tip of South America, southern beeches (genus Nothofagus) developed.
18.4 Species–Area Relationships
The spatial distribution of co-occurring plants depends on the dispersal mechanisms of species, rates of reproduction, competitiveness, growth and other factors. In nature, clumped distributions are particularly frequent, rather “island-like”, where patches are covered more densely, so that there are differences in abundance. Ecologists seeking the origin of such patterns need to know how many species (and individuals) are able to live in a certain area, known as the so-called species–area relationship (SAR).
Species–area relationships are an important background for understanding biodiversity. No single factor determines these relationships, and our present knowledge about how many factors there are and how they interact (e.g. random placement, minimum area effects and evolutionary independence) remains limited. Incomplete surveys in heterogeneous habitats contribute to the present difficulties in formulating a clear definition of these relationships, where several types currently exist. Further, attempts at clarifying species–area relations have sometimes been based on a rather small empirical basis for mathematical modelling. While mathematical models provide some insight into these interactions, they usually are based on initially simple equations, reflecting reality to only some degree, sometimes leading to incorrect conclusions. In contrast, holistic attempts have also been limited in their success, which have often led to superficial descriptive conclusions.
In this complex context it is most important to consider the spatial scales, which may differ with respect to the importance of the interactions that occur. The variability of scale is vast, from tiny soil crusts in an extreme desert to large species-rich tropical forests. Since long-term global vegetation surveys are well established—especially in the context of the relationship between climate and vegetation—large-scale maps and graphs are widely used and generally well accepted (Köppen 1900; Holdridge 1966). In these examples, vegetation is based on a classification of vegetation formations, such as evergreen rainforest, dry savanna and desert shrubland, which are characterised by floristic as well as structural/physiognomic features. More recently, computer simulations have led to a better understanding of the dynamics of global vegetation models, where most recently they have been used to predict species range shifts under climate change. For simplicity’s sake, often both climate and vegetation models are combined. This requires a reduction of the parameters—such as plant cover, water balance, biomass, soil carbon and many more—chosen for a single vegetation model. Such a reduction with respect to vegetation has even been proposed using only two plant functional types (Brovkin et al. 1997). At least two problems arise: there are too many parameters and the choice of parameters may always be comprehensible but still be arbitrary. Modellers are thus faced with the challenge of answering many questions: Should plant species, plant growth forms or plant functional types be taken into account? Further, what scale, grid size and time steps should be used for data collection in the field? The attempt to simplify often complicates one’s task because major uncertainties still surround the question of what variables to include. A model based on all ecologically important factors collected from the field for landscape and global spatial scales can prove troublesome when the focus is providing actual vegetation cover. Developing models that can accurately predict reliable changes for the future vegetation dynamics is extremely difficult and represents a major challenge facing the scientific community today.
However, recent developments in global ecosystem modelling have led to much more detailed representations of the world’s vegetation types, for example, the Lund–Potsdam–Jena (LPJ) Dynamic Global Vegetation Model (Sitch et al. 2003). Such models are based mainly on land surface biophysics and on plant functional types with different physiological, morphological and phenological attributes with field data evaluation on different time steps (Sect. 22.1).
18.4.1 Equilibrium Theory of Island Biogeography
Relations between the distribution and establishment of organisms and the size of areas have been analysed in particular for islands. Islands are clearly bound with a limited number of different habitats, often under relatively uniform climatic conditions. They occur in various sizes and are situated at different distances from the mainland. Therefore, they have been chosen as examples to clarify basic relations between number of species and size of area.
- 1.
How do plants establish themselves on islands?
- 2.
What limits the number of species on islands?

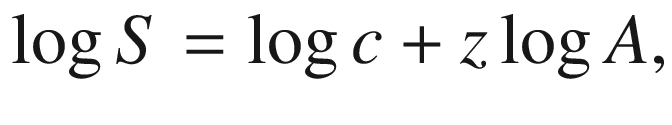
where S is the number of species of a taxon on the island, A denotes the area, z is a constant that changes little worldwide (i.e. the slope of the linear regression, when log S is plotted against log A, has values between 0.17 and 0.4), and c is a constant of proportionality and is dependent on the dimensions in which A was measured, in terms of the biogeographical area and taxonomic group.

Species-area relationships for plant species from selected Scottish islands (after Johnson and Simberloff 1974)
-
Fewer species occur on islands than in the same area on the mainland.
-
The number of species on islands increases exponentially with the size of the island.
-
The pool of species on the mainland supplies propagules.
-
The distance from the mainland is a very important determinant of the number of species on an island.
In addition, the researchers assumed that the number of species on islands depends on the diversity of habitat, and that if other islands exist between the island and the mainland, those will play an important role as a stepping stone. It was also concluded that islands possess a limited capacity for harbouring new species, which results in a sensitive equilibrium between the rate of colonisation and extinction. It should be noted that some islands may be “oceanic” islands (e.g. formed by volcanic eruptions) and as a result have never had direct contact with the mainland, or “continental” islands, where species contact has existed as a proportion of established species originating from some period of contact.
-
Larger islands have more species, also known as the “target effect” (higher colonisation rates on larger islands because they represent a larger “target”).
-
Increased distance from the mainland (supplier of propagules) results in decreased numbers of species.
-
At a constant number of species there is a continuous turnover of species due to colonisation and extinction.
-
Small islands have smaller numbers of species and higher turnover rates than large islands.
-
Islands near the mainland have more species and a higher turnover rate than those further away.
-
An island near the mainland returns to equilibrium after disturbance more quickly than one further away.


Immigration and extinction of vascular plants on volcano Rakata, Krakatau islands, between 1883 and 1989 (after Bush and Whittaker 1991)
Criticism of the MacArthur and Wilson (1967) model is also directed at the type of predictive mathematical models used. Barkman (1990) believed that to describe complex functional relations in ecosystems, only descriptive models with limited validity should be considered. The characteristics of individual plant species and their interactions among each other have not been taken into account at all in these models. It is also assumed that the increased number of species is exclusive due to colonisation; genetic evolution is not considered, nor are the aspects of species saturation.
Lomolino and Weiser (2001) suggested that species richness may vary independently of island area, especially on small islands, and they termed this the small island effect. Responsible for a higher and more predictable speciation rate on larger islands is the internal geographical isolation of very different habitats such as large river basins and high mountain ranges, which are necessary for in situ speciation. Within-island speciation rates can exceed immigration as a source of species richness, at least on islands larger than 3000 km2.
It has also been argued that phylogenetic diversification takes place on the same time scale as immigration and extinction. In this case, species richness on islands seems to be almost independent of all discussed factors. Finally, in a number of recent publications it has been highlighted more frequently that habitat heterogeneity, spatial scale and time scales (geological) are not sufficiently taken into consideration. Whittaker et al. (2008) proposed a general dynamic model (GDM) that provides explanations of biodiversity patterns by describing the relations between speciation, immigration and extinction, taking into account the life cycle of islands because climatic changes and geological tectonic events question the equilibrium model as well. In a proposed GDM, the most important variables considered remain—speciation, immigration and extinction—but are now combined with the evolutionary history of single islands and complete island archipelagos. The age of islands is also now taken into account; in particular, the geological history and the major tectonic events help to extend the thus far discussed conceptions of the equilibrium theory of island biogeography.
The theory of island biogeography also demonstrates that ecological equilibrium does not imply that ecosystems are constant and unchanging. The theory is based on a stochastic dynamic equilibrium, with a constant change in the rates of colonisation and extinction, which results in interannual variability for the actual number of species on islands and does not include speciation. Today it is becoming even more difficult to test empirically the simple assumptions of the theory of island biogeography simply due to the increasing anthropogenic influences. Although this theory has been criticised, it will be increasingly difficult to dispute, as empirical studies become increasingly harder to conduct, where the theory’s application can go beyond oceanic islands.
18.4.2 “Oceanic” and “Mainland” Islands
The results of island biogeography were formulated for islands and island groups surrounded by seawater and thus lack application for terrestrial island-like habitats. Nevertheless, attempts have been made to transfer this knowledge to the mainland and island-like habitats. These habitats are islands in lakes, mountain peaks in mountainous areas, “Inselberge” in the tropics and very small, well-isolated systems such as individual deciduous trees in a coniferous forest, caves or flower heads. Nowadays, all anthropogenic forests or biotope fragments in our managed landscapes could be interpreted to some extent as islands.
However, there are a number of commonalities to justify the application of island biogeography models to mainland islands. They have defined areas, relatively sharp borders with neighbouring habitats, are smaller than the surrounding area, and are often situated in a hostile surrounding area, at least for the taxa occurring within them. However, differences should not be overlooked, such as isolations (genetic separation) and separations (spatial separation). Usually, the distance to neighbouring islands (e.g. other forest fragments) is not very large, and the surrounding area may be hostile, but it allows at least some short-term bridging. Most of all, species turnover is faster because immigration rates are higher (e.g. from species avoiding intensive agriculture and fleeing into residual forests), as are emigration rates (e.g. because of the relative proximity of comparable neighbouring islands or sudden external disturbances).
The disappearance of a species in a habitat does not necessarily result in the extinction of the species at all. If the tree line in mountains gets lower because of a climate cooling, species will probably find refuge in the valley. Ultimately, the risk of extinction is low. With subsequent warming certain species will become established again in higher altitudes. The same principle can be applied to the diversity of species in regions that became mainland species during prolonged cold periods and then, owing to certain climatic constraints within the species, became islands again in warm periods. In this case the equilibrium theory was contrasted with the relict theory, which states that the present-day occurrence of species and communities is the result of changes in the past.
For intensively used landscapes with many small habitats a mosaic concept was developed (Duelli 1993). The number of species is explained as individual “stones” in a mosaic; within the habitat the number of species increases with the number of habitat types (even those created by humans). In a mosaic landscape there are several transitional stages (ecotones) that may be colonised by specialists. Among animals habitat diversity favours those that are dependent on a seasonal change of habitats. This concept underlines the importance of transitions and edges between neighbouring habitats.
For small forest islands, species-rich edge zones can be observed where light-demanding species occur, but not typical forest species. In near-natural ecosystems, edge zones often act as buffer zones that reduce the immigration of external species to the inside of the island, which provides the habitat for obligate forest species. In intensively used agricultural landscapes such buffer zones are often lacking and the environmental gradients to the island edges are steeper. The relationship between area and number of species may thus differ according to the proportion of the edge and the core zone. In small forest patches species composition is determined by the species of the edge zone. With increasing area, species density decreases and species typical of continuous forest zones appear. The highest diversity is achieved within this transition zone between the edge and the core. In special cases this apparently simple relation is complicated by the differences in the quality and range of influences within this zone. A distinction must also be made between natural (wind, radiation) and anthropogenic (emissions, fertilisers, mechanical disturbances) environmental factors.
The function of habitat fragment corridors or stepping stones between larger mainland islands should also be noted. These may stimulate connectivity between habitats by assisting movement across the landscape, that is, by allowing transient residence of taxa without providing a permanent habitat. At the same time they may act as refuges. It is assumed that species are able to be more successful in expanding their range when stepping stones are present. This has been empirically shown for the assisted movement of animal species in fragmented landscapes, for example, for birds (Fischer and Lindenmayer 2002), but evidence on plants remains unclear.
Plants must move within landscapes as vegetative parts (ramets), pollen or seeds, assisted by wind, water or animals (Sect. 18.2.1). Therefore, the characteristics of the spatial pattern within the mainland might be equally or even more important than that of the stepping stones or corridors. However, because plants are sessile organisms, they are much more limited in their ability to decide whether a habitat is “suitable” or “hostile”, where mobile species can respond more readily to gradients of resource availability. Thus, more recently, the simple corridor–matrix model of metapopulations and landscape ecology has been replaced by an integrative functional mosaic model, where the landscape is composed of patches of different movement and flow characteristics, which provides a much more natural interpretation of the relationships (Murphy and Lovett-Doust 2004).

Because most of us today live in managed landscapes that typically have a high degree of fragmentation, it becomes more relevant to apply the theory of island biogeography to current problems, most prominently for questions surrounding protected areas. The relation of core to edge zones in small habitats becomes extremely important. With a spatial decrease in such habitats the diversity of conditions at a site decreases linearly, but the quality decreases exponentially (Mader 1983). Species that are locally adapted to specific site conditions may go locally extinct if abrupt changes in these environments occur. In contrast, more widely distributed species, which have broader habitat requirements, may suffer limited consequences in these edge environments, unless habitat is lost due to a large or frequent disturbance. Therefore, application of the theory of island biogeography is, at the moment, only limited. Akatov (2012) discusses in more detail the shortcomings of this theory, its possible uses and recommendations for practical planning of nature conservation projects.
In spite of these known shortcomings, conservation planning is still mainly based on island theory, although established relations between the size of an area and distance (for mainland islands, the distance to the next suitable habitat) have stimulated further discussions on the minimum size of areas that should be sustained. Of course, the size of a protected area should not be calculated according to the assumptions of island biogeography exclusively. Habitats of the same or similar quality should be maintained not too far away as (perhaps only intermittently required) refuge areas, in connection with the previously mentioned stepping stones. These stepping stones are an important component of the concept of biotope connectivity for protected areas. In our agriculturally managed landscapes, such stepping stones could be small forest islands (refuge) as well as hedges, edges of fields, long-term fallow land, abandoned stone quarries or railway lines. Concepts of nature protection must incorporate, along with the protected areas, buffer zones and smaller areas of habitat fragments around the protected area, and these areas should be maximised.
Preserving a given number of species should not be the sole aim of protection measures. Large areas of mosaics of optimal and suboptimal habitats should be the primary goal of such an area. As such, there is no one-size-fits-all approach when considering the size and spatial patterns for a protected area and stepping stones (particularly the distances between stepping stones and the protected area). Each of these aspects depends on the communities one is interested in protecting and can vary substantially. Very few empirical studies have been conducted to determine the minimum distances and areas that should be used for individual groups and organisms. Currently, urgent attempts to create protected areas are based on local conditions, the plausibility of plans and the availability of land. Increasingly, the more dynamic metapopulation concept and especially the functional mosaic model (Murphy and Lovett-Doust 2004) are becoming more important to help determine these issues of protected areas. The latter not only considers the dichotomy between unsuitable and suitable habitat patches but underlines the nature of the composite landscape mosaic as a key determinant of the fate of plant populations.
18.4.3 More Models of Island Biogeography Related to the Number of Species and Area
The theory of island biogeography was taken as a paradigm. Since the 1970s and 1980s various alternative interpretations have been advanced and discussed. Those who emphasise stochastic processes explain the establishment of plant species on islands differently to those who favour determinism. All theoretical considerations are based more or less on the same factors, which are regarded as decisive for the establishment of plants; however, they are interpreted differently. As the state of knowledge currently stands, the following factors have been identified as important in this theory: the pool of propagules on the mainland, mechanisms for dispersal, distance to the island and its size, habitat characteristics, phases of succession and the conditions of competition on the island.
Connor and Simberloff (1979) assumed in their simulation model that the establishment of plants occurs stochastically without competition. They tested this model with actual data and concluded that competition does not play a role. In their interpretation, it was the parameters of dispersal that determined the success of a coloniser. Gilpin and Diamond (1982), regarding competition as the decisive factor, criticised this interpretation. They mentioned that closely neighbouring islands possess different species compositions, although an exchange of species would be possible. However, this exchange does not take place because the appropriate species niches are already occupied, where invading species will not displace species already established. In this more empirical model the explanation of diversity on islands is not possible without considering competition, so the species first present will be a contributing factor to succession and the plant community.
These and other models use only those parameters that are considered important. Often, the dimension of the temporal scale has not been considered. Time has been shown to be an important component for the establishment of different species, as pointed out by the models developed by Grime (1979) and Tilman (1988, Sect. 17.3). For the early establishment phase, a stochastic interpretation is more relevant, whereas for the later phases, a deterministic one is possible.

Pioneer spermatophytes on volcano Rakata, Krakatau islands, from 1883 to 1989. The calculation is based on the assumption of minimum turnover. On the y-axis the data for number of species observed during different expeditions are shown. Species introduced by man are not considered (after Whittaker and Jones 1994)
18.5 Problems of Pattern and Scale
Community ecology is often based on the concept of a continuum in time and space. Spatial scale has been neglected for a long time, and spatial homogeneity was often taken for granted, with spatial heterogeneity being seen as a necessary evil. However, there are different spatial levels of distribution of single plants and plant communities according to species composition and structural characteristics (e.g. forest trees or communities in small island patches or in extensive areas of several thousand hectares on large plains). Furthermore, today most landscapes are structured and determined by human activities, which influence the patterns of land and forest use depending on their size. If we deal with problems of pattern and processes of vegetation, plant coexistence and competition, then scaling issues are regarded as indispensable and fundamental to all ecological investigations because many single processes occur on different spatial scales, for example, photosynthesis (cellular scale), growth (individual scale) and species distribution (landscape scale) (Levin 1992).
Although fixing the scale along a hierarchy can be subjective, understanding the difference between fine (small) and coarse (large) processes becomes important. Furthermore, it is important to know the difference between the ecological and geographical understanding of scale. For landscape ecologists, cartographers and geographers, a small scale is a large area seen on a map with few details (e.g. landscape mosaic, array of patches, 1:100,000). In contrast, for a biological ecologist, small scale means a small part of a map showing many details (e.g. a habitat patch, 1:1000). Even patches can apply to different scales; special patterns demand special scales. For example, in applied ecology, for decision makers in agricultural planning or nature conservation, three spatial scales are most frequently used: within habitat, habitat mosaic (landscape) and macro-scale (regional, landscape mosaic).
The basis for choosing a certain scale is the degree of heterogeneity of a given environment and the specific research question being asked. There is no single “correct” natural scale. Patterns will also change across scales, where differences within a square-metre patch are likely to differ from those at the landscape scale. Further, describing an ecosystem will typically require multiple scales, ranging from the flower head of a thistle to the presence of a tree species across the Mediterranean landscape. In a fine-scale approach, species “a” and “b” may occur in different plots. In such a fine-scale approach, the diverse zonation of plant communities of a complex riverside vegetation becomes visible (Fig. 17.25). These details would be lost in a broad-scale approach using a landscape transect of a large river valley owing to necessary generalisation (Fig. 17.12).
In this context it is important to define the finest details (units), which should be visible in the chosen scale (grain), and the area chosen for investigation (extent). All three depend largely on the size of the organism or the community. In a fragmented landscape, the distance between fragments of the same quality is taken into account for the choice of an adapted scale. Grain and extent change with every change of scale. For large-scale investigations remote sensing has quite often been used; it is even useful for plant communities but would not be used at the individual scale.
Even if all these aspects are considered, we must realise that the choice of a certain scale for a certain spatial analysis is based on the individual perception of the research problem. The chosen scale seems “right” or “appropriate”, but ultimately it is still arbitrary. To obtain better answers to the comprehensive questions surrounding how environmental heterogeneity changes with scale, Wiens (1989) proposed a multiscale approach, where better insight into the interrelations between scale-dependent patterns and their causes occurs within different ecosystems. New macro-scale approaches have also been suggested, prompted by problems of global change and also based on methodological reflections coming from landscape ecology. These concern especially the study of the arrangement of larger ecological systems in space. Certain spatial patterns can only be interpreted on a larger scale, and only on such a scale can a better understanding of these patterns and ecological processes in this new field of macroecology be obtained (Gaston and Blackburn 2000). We illustrate these aspects with some examples in what follows.
Two centuries ago, Alexander von Humboldt (1807) recognised that species richness declined significantly as one follows the latitudinal gradient from the tropics to the extratropical regions. Based on the distribution of mammals, Rapoport (1975) found also a greater species diversity in the tropics, and Stevens (1992) underlined these findings and added an altitudinal gradient to this so-called Rapaport’s rule, to which also many exceptions have been noted. Latitude can be regarded as a surrogate for different environmental gradients, for example, changes in temperature, insolation, seasonality—on large scales, such as hemispheres, continents and countries (Sect. 20.3). Moles et al. (2009) studied global patterns in plant height, a decisive character of a species’ ability to compete for light, and found a close relationship between latitude and height due to a major difference in plant strategy between low- and high-latitude systems. Nobis et al. (2012) used a large-scale global approach to analyse the variation of morphological traits (especially needle characteristics). They detected a strong latitudinal correlation with phylogenetic signals due to a phylogenetic structural environmental variation among the 103 Pinus species they studied.
In Switzerland, the relationships between species richness, neophytes and their environment were analysed using a 1-km2-grid-system approach. The results suggest that climate and land use are the primary forces behind environmental change. Neophytes were found to increase in abundance with global warming, with the highest rates occurring within urban regions. Again in Switzerland, along an elevation gradient (263–3175 m a.s.l.) within 400 km2 plots the interspecific variation of 708 plant species of adult age showed clearly that temperature was the most important environmental factor in increases in age with higher elevations. Further, it was found that under warmer conditions at lower elevations the lifespan of many species was shorter. This indicates that global warming could contribute to faster species turnover, favouring short-lived species (Nobis and Schweingruber 2013).
The altitudinal gradient is a very powerful basis for testing ecological and evolutionary responses of plants and plant communities to natural environmental influences (Körner 2003). Changing temperature, together with radiation and other climatic trends, leads not only to a zonation of communities with different floristic composition but also different structurally defined communities. The phenotypic variations of pine needles (including their xeromorphic characteristics) have been assessed along a transect (ranging from lowland desert to a mountainous cloud forest) within five Canary islands as a means of describing large-scale altitudinal differentiation, and it was found that environmental changes mainly described the differences, taking into account phylogenetic influences (Lopez et al. 2008).
There are still methodological problems in the complex field of scale-based plant and plant community monitoring and in applying the results for issues such as agricultural and forest management and conservation planning. However, large-scale projects such as the Swiss Biodiversity Biomonitoring Programme are being designed to produce information about the dynamics of biodiversity at different, but especially large, scales.
18.6 Summary
-
Closely related to the development of plants and plant communities in time is their spatial distribution. The temporal dynamic leads to distribution patterns of species and plant communities. It is also the task of plant ecology to recognise, describe and explain such distribution patterns. Knowledge about the temporal and spatial dynamics of plant communities is indispensable for understanding biotic interactions within ecosystems
-
The distribution of plants starts with a mobile phase in the life cycle of all plants, with the dispersal of propagules (seeds, fruits, parts of adult plants and even whole plants). Rarely do plants just drop ripened seeds; typically, most benefit from the use of a variety of dispersal vectors such as wind, water and animals in order to distribute their propagules while trying to find suitable places for germination and final establishment
-
There is no guarantee that, following dispersal, a suitable substrate (safe site) will be found or adequate time for germination will be given. Many propagules may remain dormant for some time in a seed bank, waiting for favourable environmental conditions (light, temperature and water availability)
-
Some species, with very effective dispersal vectors and low demands of habitat conditions, are today widespread, despite continental and climatic barriers (cosmopolites). Others are bound to only small territories owing to their species-specific requirements for habitat quality, slow dispersal or young existence (endemics). Species with similar characteristics often coexist in areas with similar environmental conditions (habitat filtering). This is the basis for the area types (floristic elements, geo-elements). The interpretation of the spatial distribution of area types led to a hierarchical order, ranging from these basic areas over floral provinces and regions up to the plant kingdoms (Holarctic, Neo- and Palaetropic, Capensis, Australis and Antarctic)
-
Area types have also been an important starting point for ongoing discussions of species area relations and for the classical island biogeography equilibrium theory. On the basis of this theory, many new models were developed to acquire a better understanding of how plants become established on islands and of area limits. In some of these models islands are not only understood as small continental parts surrounded by the sea but also as a forest “island” in an otherwise tree-free area. There are two kinds of general models: (1) stochastic models, which possess some inherent randomness, and (2) deterministic models, in which the output is fully determined by the parameter values and the initial conditions (i.e. competition, habitat diversity or some degree of disturbance). However, currently our knowledge about how many factors and their interactions with each other influence species–area relations remains limited