
Most element cycles in ecosystems are open, that is, gains and losses can occur, particularly of water, carbon and nitrogen. At the scale of landscapes, resources are often imported and exported from one ecosystem to another. The export of soluble organic carbon from boreal pine forest soils in Siberia in the form of humic and fulvic acids, which co-transport heavy metals, can be clearly seen in the “browning” of the creeks and rivers, transporting the dissolved organic carbon (DOC) from these freshwater systems to the ocean. The river shown here is the Dubces, a tributary of the Yenisei in Siberia. (Photo: E.-D. Schulze).
Many definitions of biogeochemistry exist (e.g. Schlesinger 1990; Chapin et al. 2002), but here we consider biogeochemistry as the scientific study of the chemical, physical, geological and biological processes that govern the composition of the natural environment and the cycles of matter, chemical elements and energy in time and space. Thus, biogeochemistry includes the atmosphere, biosphere, pedosphere, hydrosphere, cryosphere and lithosphere. Often emphasis is placed on the study of water, carbon, nitrogen and phosphorus cycles. Biogeochemistry is a systems science, closely related to systems ecology (Chap. 13). The Ukrainian scientist Vladimir Vernadsky is often regarded as the founder of biogeochemistry as a science, since his 1926 book The Biosphere considered the physics of the Earth as a living whole.
Many different approaches are used to describe and quantify biogeochemical cycles, from observations to experiments and models (Chaps. 14 and 15). Research can be process-oriented, for example, studying decomposition or evapotranspiration, or oriented towards ecosystem budgets, for example, quantifying N inputs (atmospheric N deposition and N fertilisation) as well as N outputs (N leaching, gaseous N losses and harvests). Some of these cycles are of particular interest to multiple disciplines, such as ecology and atmospheric science, as certain processes within such biogeochemical cycles produce greenhouse gases. These gases, most importantly CO 2, CH 4 and N 2O, are released to the atmosphere where their radiative forcing contributes to anthropogenic climate change (Chap. 21). Biogeochemical processes can be driven by abiotic factors, such as UV degradation of organic matter or weathering, but also by organisms, such as earthworms contributing to organic matter mineralisation or plants fixing CO2 and symbiotic bacteria fixing N2. Interactions between autotrophic and heterotrophic organisms within an ecosystem are key to biogeochemical cycles, for example, in the rhizosphere (Chap. 11). Similarly, coupled processes, for example, the release of C, N and other nutrients during plant litter degradation, combine various namely, plants, animals and microorganisms.
16.1 Water Fluxes in Terrestrial Ecosystems
16.1.1 Water Budget at Ecosystem Scale

Water fluxes in terrestrial ecosystems. Water enters the ecosystem as precipitation or as lateral water flow (not shown), is intercepted by the canopy, and reaches the ground via stemflow and throughfall. Water leaves the ecosystem via run-off (not shown), infiltrates into the soil, contributing to seepage and groundwater recharge, but also leaves as water vapour via transpiration and evaporation

The water budget is positive if P > E + F + ΔS, for example, in areas with high rainfall. It is negative if P < E + F + ΔS, for example, when P is completely used by E or if E is fed by supplies other than P, such as irrigation. Spatially as well as temporally, the hydrological budget is highly variable, but on average, as much as 60% of total terrestrial precipitation is returned to the atmosphere by ecosystem evapotranspiration (Oki and Kanae 2006; Williams et al. 2012) (Chap. 10).

Budyko concept. a Based on annual data for precipitation (P), potential evapotranspiration (E p) and actual evapotranspiration (E), one can separate the radiative effect (driven by net radiation) from the evaporative effect (driven by precipitation) on ecosystem water vapour fluxes. The dashed lines represent the demand (E = E p) and supply (E = P) limits. The black line represents expected ecosystem water vapour fluxes. The vertical arrows depict deviations from the expected value (plus or minus). The arrow from the supply limit line to the actual data point (i.e. the term 1 − E/P) shows the percentage run-off and seepage relative to precipitation for this site. b Data from 167 ecosystem sites covering different climate types and c different ecosystem types across the globe are shown. The dashed lines in b and c represent the demand and supply limits. DBF deciduous broadleaf, EBF evergreen broadleaf, ENF evergreen needleleaf, MF mixed forest, SAV savanna including woody savanna, CSH closed shrubland, OSH open shrubland, GRA grassland, CRO cropland, WET wetland. (Modified from Williams et al. (2012))
Based on 167 ecosystem flux sites (Sect. 14.1, Chap. 14) and 764 site-years, about 93% of all the sites were found to be at or below the demand and supply limits (Fig. 16.2b, c). The remaining 7% were outside the theoretical limits (e.g. more water used for evapotranspiration than supplied via precipitation), most likely owing to measurement biases (Chap. 14) and changes in soil water storage (which is assumed to be zero in the Budyko concept) (Williams et al. 2012). About 62% of the variation in E/P across sites, that is, the fraction of precipitation returned to the atmosphere by evapotranspiration, was driven by net radiation (energy-driven), and an additional 13% was explained by climate and vegetation types (physiology-driven). This showed that despite the dominance of radiative controls on E, further vegetation type-related controls on E must be included in models of water fluxes. Interestingly, grasslands had on average a higher E/P (65%) than forest ecosystems: E/P of 56% for deciduous broadleaf forests (DBF) and 63% for evergreen needleleaf forests (ENF) (Fig. 16.2c), quite similar to croplands (E/P of 69%). This is consistent with measured leaf transpiration rates (Larcher 2003) and various strategies observed for grasses vs. trees on how to deal with water stress (Chap. 10, Fig. 10.16). However, this contrasts with the better coupling of forests (leading to higher E), with trees having deeper root systems and, thus, access to deeper soil depths (with more water; but Chap. 10, Fig. 10.12) and maybe higher leaf area index (LAI) than grasslands.
16.1.2 Water Uptake of Trees
-
The presence of roots at a certain depth does not necessarily mean that roots take up water at this depth (Chap. 10).
-
Not all roots found in the soil are physiologically active.
-
Assigning roots, particularly fine roots, to different species when studying a plant community is almost impossible. Genetic analyses have been used to determine the species, after roots have been dug out of the soil, but this approach is very laborious and expensive. More recently, near-infrared analyses of dried root material have been used to determine species identity, but this approach has a high uncertainty.

Stable isotope ratios of oxygen in precipitation, soil water and xylem water. Oxygen isotope ratios were analysed with an isotope ratio mass spectrometer in precipitation samples, soil water of eight different depths, and in xylem water of four dominant tree species (Fagus sylvatica, Picea abies, Fraxinus excelsior, Acer pseudoplatanus) in a mixed forest (Lägeren, Switzerland) over 4 years. Coloured areas along the soil profiles were modelled using a soil hydraulic model. (Data from Brinkmann 2016)

Precipitation and mean residence time of soil water. Precipitation was collected in a mixed forest (Lägeren, Switzerland) over 4 years. Mean residence time of soil water at 0–0.8 m soil depth was modelled using a soil hydraulic model based on oxygen isotope ratio measurements. The colour scale indicates the mean residence time of soil water at a given depth. Dashed lines indicate the mean residence time of soil water at 30, 60, 120, 240 and 360 days. (Data from Brinkmann 2016)
16.1.3 Evapotranspiration at Canopy and Ecosystem Scales
Evapotranspiration of ecosystems is both energy-driven and physiology-driven, as shown in the preceding section. Different processes are involved: transpiration via stomata and evaporation from surfaces. Ecosystem water vapour fluxes are thus composite fluxes since evapotranspiration can also originate from different surfaces: from soils and from vegetation (wet leaves, branches and stems). Sometimes a different partitioning method is used, canopy vs. subcanopy layer, with the latter including soil and understorey vegetation. This means that attention needs to be paid to distinguishing different units (water vapour flux in mmol per m2 leaf area or per m2 ground area) and different spatial scales (leaf, soil, canopy, ecosystem; for leaf transpiration, Chap. 10).

-
Available solar energy for supplying the energy needed for the vaporisation of water.
-
Precipitation input, water vapour pressure deficit of air, soil moisture, soil structure, supplying water and controlling water uptake.
-
Roughness of surface (soil, canopy, ecosystem), which determines the coupling of the canopy to the atmosphere.
-
Ecosystem structure, for example, fraction of bare soil, stand architecture, LAI, affecting energy budget of the ecosystem.
-
Plant structural variables such as leaf area, and the leaf angle, affecting the absorption of incoming radiation.
-
Leaf ecophysiology such as leaf conductance
-
Spectral characteristics of surfaces, which determine the sensible heat flux.

Evapotranspiration as coupled energy and water flux. Energy a and water b fluxes are tightly coupled since the evaporation of water needs energy. SWnet = net shortwave radiation (SWin − SWout), LWnet = net longwave radiation (LWin − LWout), H2O = atmospheric water vapour, CO2 = atmospheric CO2. For simplicity other greenhouse gases are not shown in the figure. SH = sensible heat flux, G = soil heat flux, P = precipitation, E = evapotranspiration, F s = surface run-off, F g = seepage. The sum of F s and F g represents the term F in Eq. (16.1). Changes of energy within the soil and changes in water content within the soil are omitted. (Modified from Seneviratne et al. (2010))


Analytical representation of water vapour and CO2 fluxes in a terrestrial ecosystem. Both water vapour and CO2 fluxes are affected by abiotic and biotic drivers. See text for explanations and derivation of equations
where the coefficient s (in Pa K−1) describes the change in saturation vapour pressure with temperature, D is the saturation vapour pressure (in Pa), ρ is the density of air (1.204 kg m−3 at sea level), c p denotes the specific heat capacity of air (1012 J kg−1 K−1), and γ (Pa K−1) denotes the psychrometric constant (66.1 Pa K−1) (Chap. 9). Both conductances G W and G H (in m s−1) describe the entire pathway between the leaves and bulk air, including stomatal, cuticular and boundary layer components at the ecosystem level. The soil heat flux G is rather small, typically 2% of R n in dense canopies, increasing to about 30% in very sparse canopies.



However, to be closer to reality, currently also “two-leaf models” are used, which account for different behaviours of sunlit and shaded foliage within a canopy (Liu et al. 1997) (Chap. 15). Those interested in mathematics should read the work by Raupach and Finnigan (1988) with the remarkable title “Single-layer models of evaporation from plant canopies are incorrect but useful, whereas multi-layer models are correct but useless”. Fortunately, since this publication, science and particularly model development have progressed, and optimisation theory and remote sensing approaches are used to describe canopies in more complex ways (e.g. Buckley et al. 2013; Peltoniemi et al. 2012).
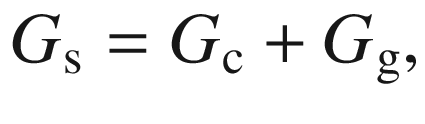
Surface conductance G s approaches canopy conductance G c when the leaf area increases because soil evaporation becomes negligible in dense canopies (LAI > 3). It should also be noted that G s is not equal to leaf conductance g L because it includes not only the surfaces of vegetation in the ecosystem, but also the surface of the soil (Fig. 16.6) (Schulze et al. 1994).


 = mean leaf conductance in stratum i
and LAi = leaf area in stratum i.
= mean leaf conductance in stratum i
and LAi = leaf area in stratum i.Because carbon dioxide and water vapour fluxes of a canopy are tightly coupled, we included carbon dioxide assimilation of the canopy A c in Fig. 16.6 as well. A c is determined by leaf net photosynthesis, which is controlled by the CO2 gradient between the ambient air and the leaf intracellular air space (= c a – c i), the leaf conductance g L, and foliar nitrogen nutrition, scaled up by canopy LAI (modulated by incoming radiation and leaf arrangement, for example, leaf angles, clumped needles and so forth).

Relationships between maximum canopy conductance (G c from vegetation only) and surface conductance (G s from soil and vegetation) and their relation to LAI. The continuous lines show the corresponding surface conductance, the hatched lines the canopy conductance. The shaded areas between the lines show the conductance of moist soil G g. Two different sets of curves are given, based on maximum stomatal conductances of 4–12 mm s−1, the range found on Earth. (After Schulze et al. 1994)

Diel courses of the different components of the energy budget and corresponding temperatures over a medium intensively managed grassland in Switzerland (Früebüel, 1000 m asl, 30 May 2009). a The continuous line shows the net radiation R n, the broken lines show λE, the latent heat flux, H the sensible heat flux, and G the soil heat flux. ΔQ represents the lack of energy balance closure, for example, the change in heat storage in the vegetation. b The continuous line shows the temperature at the top of the sward T surface, the broken lines show the air temperature T air and the soil temperatures T s at five soil depths (soil depth given as subscript: 0.01, 0.10, 0.15, 0.40 and 0.95 m). SR sunrise, SS sunset. (Data from W. Eugster)
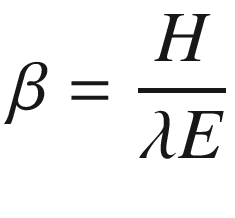
Comparison of albedo, sensible and latent heat fluxes as well as Bowen ratios across ecosystem types
|
Evergreen forests |
Deciduous forest |
Grasslands |
Croplands |
|
|---|---|---|---|---|
|
Albedo (%) |
10 ± 2 |
13 ± 1 |
20 ± 2 |
17 ± 2 |
|
Sensible heat H (%) |
44 ± 10 |
25 ± 7 |
44 ± 15 |
19 ± 4 |
|
Latent heat λE (%) |
46 ± 10 |
62 ± 7 |
46 ± 15 |
64 ± 4 |
|
Bowen ratio (H/λE) |
0.5 to 1 |
0.25 to 0.5 |
highly variable |
0.25 to 0.5 |
It is interesting to note that the Bowen ratio increases when temperate ecosystems are converted to urban settlements, approaching values similar to deserts. In contrast, Bowen ratios decrease with irrigation, indicating higher water vapour and lower sensible heat fluxes due to evaporative cooling. Another useful ratio describes the fraction of available energy (R n – G) used for latent heat λE, thus λE/(R n – G), which is more intuitive and often more useful than the Bowen ratio.
16.1.4 Imposed and Equilibrium Evapotranspiration of Leaves and Canopies
- 1.
The leaf or canopy is well coupled to the atmosphere. This is the case for small leaves or isolated plants. Here, the boundary layer around the evaporating surfaces is very small, so the boundary layer conductance is very large, and the transfer of heat and mass (water vapour) is very efficient. This means that leaf or canopy temperatures approach air temperatures depending on the radiative input, and physiological controls dominate evapotranspiration. Thus, Eq. (16.4) is reduced to describe the imposed evaporation E imp:

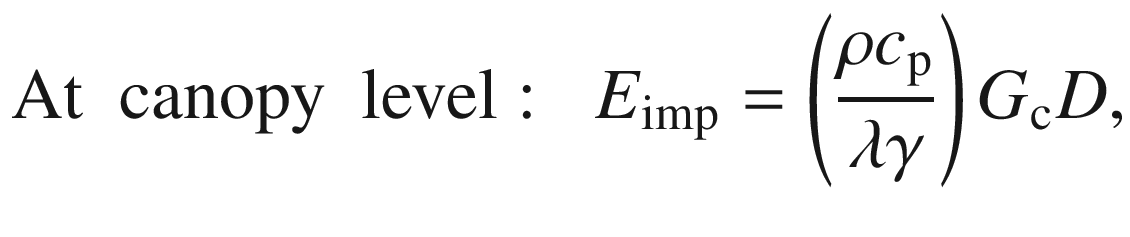
-
where g L or G c describes the corresponding physiological conductance of the leaf or the canopy. Owing to the full coupling, the conditions of the atmosphere are “forced or imposed” on the leaf or canopy, so that evapotranspiration is linearly related to conductance and water vapour deficit (which in turn is affected by conductance).
- 2.
The leaf or canopy is not well coupled to the atmosphere. This is the case for large leaves, very short vegetation (short lawn), conditions with still air (no turbulence) or within a greenhouse. Here, the boundary layer around the evaporating surfaces is very large, so the boundary layer conductance is very small, and the transfer of heat and mass (water vapour) is very poor. This means that the energy supply R n dominates evapotranspiration. Thus, Eq. (16.4) is reduced to describe equilibrium evaporation E eq:

For both leaf and canopy evapotranspiration, water vapour loss is in equilibrium with the available net incoming radiation R n, independent of leaf or canopy conductance. Such conditions exist for example on an even lawn. Leaf stomata may be wide open, but the canopy will lose little water because the transport of water vapour through the boundary layer does not take place. Thus, evapotranspiration saturates the boundary layer, and D will approach zero. Water vapour transport to the atmosphere is only enhanced if the temperature of the surface rises as a consequence of radiation. For large crop areas, actual evapotranspiration is about 26% larger than E eq based on Eq. (16.12), so a factor 1.26 is introduced into the nominator (Priestley–Taylor coefficient) to increase the estimated evapotranspiration rate E eq. The discrepancy arises because turbulent mixing in and above the field prevents the boundary layer from becoming fully saturated, so D is larger than zero.
Since E eq is purely energy-driven, one can also interpret E eq as potential evaporation (no physiological effect via transpiration). Physically, E eq represents simply the water vapour flux from an open water surface that has the same surface temperature as a leaf or a canopy fully supplied with water, under conditions when the atmosphere is fully saturated with water vapour (D = 0). However, there are many different ways to calculate potential evaporation (for details, see climatology textbooks).






Relationship between height of a canopy and its decoupling from the atmosphere. a Dependence of decoupling factor Ω on height of vegetation as a measure of roughness of surface (data from Kelliher et al. 1995). b In boreal forests, the ratio of latent to sensible heat flux is affected by the form of the tree canopy. Picea obovata x excelsa (narrow crown) is more densely covered with needles and therefore warms more than Picea excelsa (broad crown) or Betula pubescens. The thick ground vegetation with Vaccinium myrtillus does not have a very rough surface and thus warms more strongly. Gutulia, central Norway. c Canopy of a Eucalyptus marginata stand with an average height of 100 m in south-western Australia. Eucalyptus marginata reaches a height of 140 m. In contrast to boreal forests, the leaf surface is almost uniformly distributed in the canopy, although Eucalyptus forms leaf bundles at the ends of branches. Picture taken from the Gloucester tree near Pemperton. (Photos E-D Schulze)
Decoupling can also be achieved by specific morphological adaptations at the leaf level, which reduce the boundary layer around the leaves. For example, leaf petioles with an elliptic, flattened cross section lead to leaf movements even at very low wind speeds. The fast-growing species Populus tremula has “shivering” leaves (Latin name tremulus = trembling) already in light breezes, decreasing the leaf boundary layer, thus increasing the boundary layer and leaf conductances and, in turn, leaf gas exchange (Sect. 9.3 in Chap. 9). Thus, E imp dominates leaf and canopy E, being strongly driven by g L and D (Eq. 16.11a). Growing on moist soils, this species can afford high transpirational losses due to high leaf conductances to enhance CO2 uptake and, thus, growth.
Box 16.1: Techniques to Measure Transpiration and Evapotranspiration at Different Scales
-
Porometer: Contains a sensor head onto which a leaf is pressed. The changes in air humidity and CO2 concentration (facultative) over time are measured as the basis for flux calculations. Advantage: simple to use. Disadvantage: The leaf response is measured under artificial conditions.
-
Cuvettes: These are controlled chambers in which a leaf is enclosed. Temperature and humidity can be controlled, so it is possible to determine plant responses to specific conditions. Advantage: Experiments can be conducted in the field. Disadvantage: The measured transpiration rate does not correspond to the transpiration rate under undisturbed conditions.
-
Xylem flux: The water in the xylem is heated at a constant rate and the distribution of temperature in the stem is measured (Granier method). Advantage: It allows measurements of the natural rate of transpiration of plants. Disadvantage: The method is best suited for trees. Sapwood area needs to be known. Extrapolation to the whole vegetation surface is difficult.
-
Point dendrometers: The girth of stems is measured continuously, with a small pin touching either the bark or the xylem. Measurements can be automated, frequency as high as 10 min. Stem radius changes are calculated. Advantage: Continuous, high-precision measurements. Cheap. Disadvantage: The method works best with trees. Secondary growth as well as shrinking and swelling due to water dynamics affect stem diameters; both processes need to be distinguished to estimate tree water deficit. (Zweifel et al. 2016)
-
Eddy covariance method: The turbulent flux in the air above an ecosystem is derived from measurements of vertical wind speed and gas concentration in air parcels moving past the respective sensors. Advantage: Integrated measurement of fluxes for whole ecosystem (0.5–4 km2), high temporal resolution (20 Hz, averaged over 30 min). Disadvantage: Needs stationary turbulent atmospheric conditions. Lots of post-processing needed. Assumes negligible advection. Expensive (Aubinet et al. 2012)
-
CBL budgeting (CBL is the convective boundary layer of the atmosphere): The lower layer of the atmosphere is taken as a closed box (Lloyd et al. 2001). During the day, changes in gas concentration at different heights in this idealised box are measured from an airplane and fluxes within the box from the surface below can be estimated at landscape scale (50–100 km2). Advantage: Quantification of flux budgets over heterogeneous landscapes. Disadvantages: Technically and meteorologically demanding. Limited to particular meteorological conditions (typically single nice weather days). Expensive
16.1.5 Responses of Terrestrial Ecosystems to Drought
Biogeochemical processes in ecosystems react to changes in environmental conditions just like physiological processes in leaves and individual plants. Using the appropriate measurement techniques for the scale under study, here the ecosystem scale (Box 16.1), one can quantify these changes and compare magnitudes of process rates and responses of different ecosystem types to the same driver. Moreover, one can study the interactions of different biogeochemical processes, like evapotranspiration (ET) and gross primary production (GPP). The ratio of GPP to ET (sometimes called ecosystem water-use efficiency) also provides insights into different survival strategies, that is, how the ecosystem reacts to environmental stress such as a severe drought. This is of particular interest since extreme events like droughts are expected to increase with anthropogenic climate change (Chap. 21).

Relationship between water vapour fluxes and gross primary production of grassland and mixed forest. The slope of the curves represents the ratio of GPP to ET of a a grassland (Oensingen, Switzerland) and b a mixed forest (Lägeren, Switzerland) over the course of 2 years. The year 2010 represents an average year for both sites, while 2011 was a year with a strong spring drought. (Wolf et al. 2013)
These different behaviours of grasslands and forests were supported by combining flux measurements and models at the next larger scale, the region. For the combined heatwave and drought in summer 2006 across Europe, grasslands showed much higher evapotranspiration rates at the beginning of the heatwave and drought than forests (Teuling et al. 2010). This resulted in a cooling of the atmosphere over grasslands but a heating over forests. Only longer into the extreme event, when grasslands started to wilt and die off, but forests still continued to transpire (although at a low rate), did this result in a cooling of the atmosphere over forests at later stages of the heatwave and drought. This shows how different ecosystems differ in their responses and feedbacks to changing environmental conditions, in this case to the atmosphere (Chap. 23).
16.2 Carbon Fluxes in Terrestrial Ecosystems
16.2.1 Carbon Pools and Fluxes in Terrestrial Ecosystems

Carbon pools and fluxes in terrestrial ecosystems. Carbon enters the ecosystem as carbon dioxide via photosynthesis and leaves the system as carbon dioxide via autotrophic (root, stem, foliage) and heterotrophic (microbial, soil fauna) respiration. Further carbon losses occur as leaching of dissolved organic and inorganic carbon (DOC and DIC, respectively) as well as via erosion. Additional losses result from harvests and fires
One variable often used to address ecosystem carbon budgets is net primary production (NPP), in g C m−2 year−1, defined as GPP minus respiration (Sect. 12.5 in Chap. 12 for leaf and plant levels and Chap. 23 for global change impacts). Thus, it includes, for example, production of fine roots, root exudation and emission of BVOVs for defence (Sect. 16.2.4). NPP constitutes about 50% of GPP (ranging from 30% to 80%) (Amthor 2000; DeLucia et al. 2007) and can be determined for above-ground and belowground tissues (ANPP and BNPP, respectively). Moreover, allometric relationships (i.e. statistical relationships used to predict tree biomass) relate BNPP to total NPP. For example, for trees BNPP/NPP = 0.3, and for grasses BNPP/NPP = 0.5. These numbers are global means and thus do not account for species differences (Sect. 22.2 in Chap. 22). Moreover, most NPP estimates given in the literature are ANPP estimates only, neglecting below-ground production but also herbivory and other processes like exudation and BVOC emissions.
Thus, NPP should not be interpreted as yield, harvest or above-ground biomass production over time (e.g. ANPP per growing season, cropping time, year). Two examples illustrate this misconception: Farmers consider grain production as yield but are not necessarily interested in stem and root growth; foresters are mostly interested in cumulative stem growth and increase in wood volume at time of harvest but not in foliage growth or even stem mortality until harvest. Moreover, thinning and harvest residues are clearly part of NPP but not considered in assessments of net increment changes in forests. Both examples illustrate that the harvestable yield is only a small fraction of NPP.
Standing biomass, above-ground net primary production (ANPP), and leaf litter production in different ecosystem types
|
Ecosystem type |
Biomass (kg C m−2) |
ANPP (kg C m−2 year−1) |
Leaf litter (kg C m−2 year−1) |
|---|---|---|---|
|
Tropical rainforest |
20–32 |
3–10 |
0.5–1.4 |
|
Temperate deciduous forest |
5–30 |
0.2–1.2 |
0.1–0.6 |
|
Temperate coniferous forest |
15–75 |
0.4–1.3 |
0.1–0.6 |
|
Boreal coniferous forest |
8–10 |
0.3–0.4 |
0.1–0.5 |
|
Savanna |
1–2 |
0.4–0.6 |
0.1–0.4 |
|
Grassland |
0.2–2.2 |
0.1–1.0 |
<0.1–1.0 |
|
Cropland |
0.5 |
0.3–0.5 |
0.1–0.2 |
|
Desert |
0.2–3 |
<0.1–0.5 |
<0.1–0.2 |
|
Tundra |
0.1–2 |
<0.1–0.2 |
<0.1 |
|
Ecosystem type |
Roots |
Fine roots |
Leaves or needles |
|||
|---|---|---|---|---|---|---|
|
Total (kg m−2) |
Mass (kg m−2) |
Length (km m−2) |
Root surface index (m2 m−2) |
Mass (kg m−2) |
Leaf area index (m2 m−2) |
|
|
Tropical rain forest |
4.88 |
0.57 |
4.1 |
7.4 |
2.5 |
11 |
|
Temperate coniferous forest |
4.40 |
0.82 |
6.1 |
11.0 |
1.3 |
9 |
|
Temperate deciduous forest |
4.14 |
0.78 |
5.4 |
9.8 |
0.4 |
7 |
|
Boreal forest |
2.92 |
0.60 |
2.6 |
4.6 |
1.8 |
11 |
|
Shrub vegetation |
4.82 |
0.52 |
8.4 |
11.6 |
0.4 |
8 |
|
Savanna |
1.40 |
0.99 |
60.4 |
42.5 |
0.9 |
3 |
|
Temperate grassland |
1.56 |
1.51 |
112.0 |
79.1 |
0.6 |
3 |
|
Tundra |
1.25 |
0.96 |
4.1 |
7.4 |
0.4 |
2 |
|
Desert |
4.13 |
0.27 |
4.0 |
5.5 |
0.1 |
<1 |
|
Average |
3.28 |
0.78 |
||||
-
Sequential root biomass sampling.
-
Ingrowth cores: mesh bags filled with root-free soil, placed into the rooting zone, and collected after some time to quantify the new roots grown into the cores.
-
Minirhizotrons: cameras inserted into the soil to monitor root growth with transparent tubes.
-
Stable isotope labelling: tracing the fate of 13C–labelled photoassimilates into root growth.
-
Radiocarbon dating based on bomb-carbon: 14C created by above-ground nuclear tests done in the 1950s and 1960s (also called bomb-carbon), recovery of bomb-carbon in tissues, compounds or ecosystem compartments, relationship to decreasing atmospheric 14C background values and thus to the age of the C analysed.
Each method has its own uncertainties, but generally sequential coring and minirhizotron estimates seem to overestimate turnover, while radiocarbon analyses seem to underestimate turnover (Gaul et al. 2009). Consequently, estimates of mean life span for the same fine roots might differ. In temperate forests, life spans of typically <1–2 years were found based on sequential coring and minirhizotron methods. However, fine root life spans were much larger, up to 8 years (top soil) and 18 years (deeper soil horizons), based on radiocarbon analyses (Gaudinski et al. 2001). However, radiocarbon estimates can be biased, depending on which carbohydrate substrates with which age (i.e. time since bomb tests) are used for fine root production. Clear evidence was provided by Vargas et al. (2009) using radiocarbon analyses in five tropical forest stands before and after Hurricane Wilma hit. Fine roots grown into ingrowth cores installed after the hurricane were estimated to be 2–10 years old. Thus, trees had remobilised stored, that is, old, carbohydrates to supply new fine root production and did not use recently assimilated carbon. This made new roots look much older! Independently of the method used, most studies find thicker and deeper roots being older than fine roots in the top soil. Root turnover differs among different ecosystem types. For example, fine root turnover in grasslands is typically much greater than in forests, that is, the life span of fine roots in grasslands is much shorter. In comparison, leaves and needles in temperate forest ecosystems have a life span of 1–10 years (Pinus aristata even 30 years). Wood can remain at a plant >100 years (oldest living trees: 5000 years, Pinus longaeva, Bristlecone pine), while SOM is often >100–1000 years old.

Processes and organisms contributing to soil respiration in the form of soil CO2 efflux to total ecosystem respiration. Turnover rates and, thus, residence times of the substrates differ among the different component fluxes, as do the main drivers. (Kuzyakov and Gavrichkova 2006)
Overall, the most important driver of soil respiration is canopy assimilation. This can be seen when above-ground biomass is cut, for example, in managed grasslands, while abiotic conditions like soil temperature and moisture remain unchanged. Soil respiration drops within a couple of days and only resumes when above-ground biomass, and thus LAI, is regrown. Högberg et al. (2001) used stem girdling in a northern pine forest to show for the first time the strong coupling of canopy photosynthesis to below-ground respiration (Sect. 14.2, Chap. 14). Girdling is the removal of the phloem in the bark of trees, which interrupts the supply with carbohydrates assimilated in the canopy to the root system. Depending on the starch pool in the roots, the drop in soil respiration occurred faster (low starch pool, end of season) when trees were girdled later in the year than when girdled in spring (still high starch pool, beginning of season). Similarly, drought slows down carbon allocation below-ground, affecting soil respiration negatively (Rühr et al. 2009). Thus, overall, soil respiration scales positively with GPP, NPP, LAI, litter production and carbohydrate supply below-ground. This affects both root-rhizosphere respiration (RR) and MR, which are tightly related to each other as well (Bond-Lamberty et al. 2004). Changes in phenology, co-occurring with changes in environmental conditions, thus affect soil respiration in mixed deciduous forests too (Ruehr and Buchmann 2010). During the growing season with full canopy cover, RR is greater than microbial soil respiration, contributing on average 50–60% to total soil respiration. However, during the dormant season, this contribution of RR drops to about 30%, with microbial soil respiration dominating the overall soil CO2 flux.

Functional relationships of soil temperature with soil respiration (SR), microbial (MR) and root-rhizosphere respiration (RR). Fluxes were measured in a mixed deciduous forest during the dormant seasons (no tree foliage; 2006/2007 and 2007/2008) and the growing season (full canopy; 2007). SR was measured on grown forest soil. Using mesh bags, roots were excluded from the bags, and MR could be measured separately. RR was calculated by difference (RR = SR − MR). (Ruehr and Buchmann 2010)
Soil respiration fluxes across different ecosystem types
|
Ecosystem type |
Soil respiration (g C m−2 year−1) |
|---|---|
|
Tropical rainforest |
666–1520 |
|
Temperate deciduous forest |
304–1414 |
|
Temperate coniferous forest |
250–1300 |
|
Boreal coniferous forest |
120–550 |
|
Savanna |
380–900 |
|
Grassland |
132–1988 |
|
Cropland |
224–1410 |
|
Desert |
184–300 |
|
Tundra |
29–95 |
16.2.2 Decomposition and Stabilisation of Organic Matter in Terrestrial Ecosystems
-
Decay, which describes the biotic breakdown of dead plant organic matter (also called necromass) into smaller pieces (degradation) by soil fauna and microorganisms and the related mass loss (Sect. 20.4 in Chap. 20).
-
Mineralisation, which describes the release of nutrients in inorganic forms from organic matter, including the release of CH4 or CO2 (Sect. 16.2.1).


Degradation of leaf litter from beech over time. a Initially, the easily available carbohydrates are consumed. Then lignin decomposition starts (after Berg and Matzner 1997). b Parallel with the decomposition of dry matter, decomposition of N-containing substances occurs. (Data from F. Cotrufo)

Decomposition of dead wood after a fire in a Siberian pine forest. The mean residence time is only about 40 years, despite the cold climate. This is because of repeated fires at ground level. As these fires only burn the outside of the wood, single dead trunks can be traced back 400 years. (Wirth et al. 2002)
The stabilisation of organic matter, that is, the formation and protection of SOM, has received a lot of attention over the last decade. Stabilisation is related to chemical changes of decomposition products mediated by soil fauna (e.g. earthworms that change the chemistry of the organic residue) and microorganisms, but also to physical protection, for example, by the soil matrix, by association with minerals or occlusion in soil aggregates. Both factors can reduce the chance that organic matter will be decomposed and thus lead to the persistence of organic matter in the soil over long time periods (Schmidt et al. 2011; Cotrufo et al. 2013), contributing to long-term carbon (and nutrient) storage in soils. For example, newly formed microbial polysaccharides (bacterial slime) bind to clay and silt fractions in the soil and are thereby stabilised against further decomposition. Thus, the clay content determines the ability of soils to store C (Bird et al. 2001). In stark contrast to the initial steps of decomposition, during stabilisation, the molecular structure of the organic matter or the recalcitrance of organic matter as a substrate for decomposition is only marginally important. Even “easily decomposable” sugars can persist in the soil for decades when protected (or immobilised in constantly active microbial biomass), and even “recalcitrant” lignin and plant waxes can be decomposed at rates higher than the bulk soil when conditions are right. Similarly, the relevance of humic substances as very stable SOM fractions, formed de novo during decomposition, needs to be revisited. Inferred from the classical extraction methods in soil chemistry, the amount and relevance of humic substances have been largely overestimated. Their new formation is no longer considered quantitatively relevant for humus formation in soils. Nevertheless, most soil carbon models still use the structure and inferred decomposability of organic matter to drive their decomposition models (Chaps. 15 and 22). Instead, fire-derived organic matter has been identified as being highly relevant and can make up to 40% of total SOM in many forest and grassland soils (Box 16.2). Deep soil layers are still a black box, although globally they store more than 50% of total soil carbon pools.
Box 16.2: Black Carbon and Terra Preta Soils

Black carbon in terra preta soils in central Amazonia. a Putative structure of black carbon. In charcoal and soot, cyclic hydrocarbon molecules recrystallise into a condensed lattice of benzene rings (Gleixner et al. 2001). b Ferralsol, an abundant, but infertile, soil type in the tropics. c Terra preta, a black-earth-like anthropogenic soil in central Amazonia, also found in West and South Africa. (Photos B Glaser)
In the central Amazon, “islands” with highly fertile soils, called terra preta, have been recorded, surrounded by very infertile soils, often ferralsols (Fig. 16.16b, c). These fertile soils contain about 3 times the amount of SOM, about 70 times more charcoal, higher nutrient content and a better nutrient retention capacity than their surrounding soils (Glaser 2007). These soils were formed in pre-Colombian times, about 7000 to 500 years BP, and have been used for agriculture ever since, so they are considered anthropogenic soils. The origin of the black carbon (also called biochar) is not entirely clear, but natural forest fires do not sufficiently explain the high black carbon content. Anthropogenic activities such as low-heat smouldering fires as used for cooking or spiritual uses have been proposed as source for the biochar. Higher microbial activities are found in terra preta soils as well as after charcoal additions to infertile ferralsols, probably due to the porous structure of charcoal, providing habitats for soil microorganisms. In addition, biochar is electrically conductive, so it can affect electron transfer processes by functioning as an electron shuttle, increasing, for example, N2 fixation by free-living prokaryotes (Kappler et al. 2014). After the original combustion product has been oxidised on the edges by microorganisms in the soil (Glaser et al. 2002), black carbon also adds a cation exchange capacity to soils and, thus, a high nutrient retention capacity. Currently, the application of charcoal for sustainable agriculture is recommended.
16.2.3 Net Ecosystem Production and Net Biome Production



Carbon budget of forest ecosystems. The initial process is gross primary production (GPP), which corresponds to gross photosynthesis. About 50% of the photoassimilates are used for net primary production (NPP); the rest is available for growth respiration and maintenance respiration. Accounting for heterotrophic respiration from litter, coarse woody debris (CWD) and from soils leads to net ecosystem production (NEP). If further processes are considered that remove or add C from or to the system, one talks about net biome production (NBP). CWD coarse woody debris, SOM soil organic matter, BC black carbon, Rh heterotrophic respiration, Ra respiration of autotrophic plants (After Schulze et al. 2000)
To know when an ecosystem is a carbon sink or a source, the net balance between gross photosynthesis and ecosystem respiration needs to be known. The diurnal courses of NEE clearly show when a particular process dominates. During the night, only respiration occurs. During the day, photosynthesis can be compensated by respiration (often the case in winter) or photosynthesis can overcompensate respiration (during most of the growing season). Thus, depending on the time of day and the day of the year, the ecosystem acts as either a CO2 source (nighttime, winter) or a CO2 sink (daytime, growing seasons). This general pattern occurs throughout the year, but environmental conditions, phenology and management determine the relationship of CO2 loss to uptake and, thus, the daily NEE flux.

Annual and diel courses of net ecosystem CO2 exchange of an intensively managed grassland over 2 years. Net ecosystem CO2 fluxes in μmol CO2 m-2 s-1 were measured with the eddy-covariance method at Chamau, Switzerland, located 400 m asl. a Net ecosystem CO2 fluxes are given in the micrometeorological sign convention over 24 h for the 2 years 2006–2007. b Seasonal courses of cumulative CO2 fluxes differ between the 2 years. Frequent grass cuts (6–7 per year) and subsequent manure applications result in the typical zigzag pattern of CO2 fluxes in grassland. (Data from M. J. Zeeman)
If an ecosystem does not experience additional exports and inputs during the year (e.g. by management or DOC fluxes), the final value of the cumulative NEE curve gives an estimate of the ecosystem carbon sink. This is often the case in forests, which are not managed on an annual basis. Evergreen forests can exhibit small sink activities also over winter, while sink activities in deciduous forests start with the leaf-out of the understorey vegetation (e.g. Allium ursinum, wild garlic, in temperate beech forests) and show peak NEE fluxes when the tree canopy is fully developed. Thus, phenology is an important driver of NEE and NEP. The longer the growing season, the higher the NEP: deciduous broadleaf forests increase their NEP by 5.6–5.8 g C m−2 per day of the growing season, evergreen needle-leaf forests by 3.4 g C m−2 day−1, savannas by 3.7 g C m−2 day−1 and grasslands and croplands by 7.9 C m−2 day−1 (Churkina et al. 2005). Climate variables have large effects on NEE, but also on GPP and R e. Both component fluxes depend primarily on light but increase with temperature and precipitation until limitations set in (heat or freezing stress, drought). This is true not only of the current year’s weather but also of the previous year’s weather, since carbon allocation and storage drive bud establishment and growth the following year. Furthermore, disturbances, either slow or sudden, affect ecosystem CO2 fluxes. N deposition was shown to increase the NEP. Fire frequency and intensity affect both GPP and R e. Drought and heatwaves affect GPP more negatively than R e, shifting ecosystem NEE towards respiratory losses and even releasing CO2 from SOM, sometimes worth several years of C sequestration (Ciais et al. 2005). Old forests still sequester carbon (Sect. 14.1, Chap. 14), although sometimes at lower rates than mature forests. For further examples, please see Baldocchi (2008, 2014).
NEP, GPP, R e and NBP estimates across ecosystem types
|
Ecosystem type |
NEP (g C m−2 year−1) |
GPP (g C m−2 year−1) |
R e (g C m−2 year−1) |
NBP (g C m−2 year−1) |
|---|---|---|---|---|
|
Tropical rainforest |
403 ± 102 |
3551 ± 160 |
3061 ± 162 |
NA |
|
Temperate deciduous forest |
311 ± 38 |
1375 ± 56 |
1048 ± 64 |
For Europe: |
|
Temperate coniferous forest |
398 ± 42 |
1762 ± 56 |
1336 ± 57 |
75 ± 20 |
|
Boreal coniferous forest |
131 ± 79 |
973 ± 83 |
824 ± 112 |
NA |
|
Savanna |
360 ± 17 |
1380 ± 70 |
1020 ± 20 |
200 |
|
Grassland |
247 ± 67 |
2296 ± 80 |
2104 ± 32 |
104 ± 73 |
|
Cropland |
240 ± 113 |
1246 ± 248 |
1006 ± 222 |
−11 ± 33 |
|
Desert |
28 ± 16 |
170 ± 39 |
143 ± 23 |
NA |
|
Tundra |
5 − 67a |
15 − 130a |
5 − 64a |
NA |
Mean residence times of entire ecosystems
|
Ecosystem type |
Mean MRT (years) |
Range MRT (years) |
|---|---|---|
|
Tropical rainforest |
14.2 |
11.6–18.2 |
|
Temperate forest |
23.5 |
18.9–30.8 |
|
Boreal forest |
53.3 |
45.4–73.4 |
|
Savanna |
16 |
12.2–22.1 |
|
Grassland |
41.3 |
32.8–54.6 |
|
Cropland |
22.1 |
17–30.1 |
|
Desert |
36.3 |
27.6–49.9 |
|
Tundra |
65.2 |
44.7–78.0 |

Conceptual model of stable carbon isotope ratios δ13C and leaf carbon isotope discrimination Δ (in per mil compared to an international standard) in a tropical forest in French Guiana. Foliage values (circles) are given for different heights within the canopy. Soil and litter (squares) and soil-respired CO2 (diamond) signatures are given. Canopy CO2 values (lines) are given for night- (solid) and daytime (dashed). (Buchmann et al. 1997)
Moreover, the δ13C in foliage integrates over the entire lifespan of a leaf, while δ13C litter integrates over all species currently present in an ecosystem. In turn, δ13C of SOM integrates over current and past vegetation. This is particularly interesting when vegetation had changed from C 3 to C 4 vegetation or vice versa, since carbon isotope discrimination differs strongly between these two photosynthesis types (Sect. 12.2, Chap. 12). Thus, δ13C of SOM within a soil profile can give information about the dominant vegetation, forest or (C4) grassland, over time, particularly when the age of SOM is known. The difference between the δ13C of atmospheric CO2 entering the ecosystem for GPP and the δ13C of respired CO2 of ecosystems, the so-called isotopic disequilibrium, is an important piece of information for global inverse atmospheric models that are used to estimate global sinks and sources.
16.2.4 Fluxes of CH4 and Other Biogenic Volatile Organic Compounds
Although CO2 dominates the discussion about the carbon dynamics of ecosystems, CO2 is not the only carbon-containing gas being exchanged between ecosystems and the atmosphere: the exchange of methane (CH4) and other BVOCs are highly relevant for many ecosystems as well.

CH4 emissions from soils, mediated by vascular plants (Le Mer and Roger 2001)

Methanol emissions from three temperate grasslands in Austria and Switzerland measured using micrometeorological techniques. Large peaks can be seen 1–3 days after the corresponding management events. Different symbols depict different sites. (Wohlfahrt et al. 2015)
16.3 Nitrogen Fluxes in Terrestrial Ecosystems

Nitrogen cycle in terrestrial ecosystems with a focus on plant–microbe interactions (Modified from Schulze (2000)). Not all microbial processes are depicted



Changes in oxidation states of N during N transformations and impacts of N2 fixation. a Functional groups of microorganisms in biogeochemical nitrogen cycle. Blue arrows indicate reactions that occur within a single organism. Small black arrows are intermediate products. The figure also shows the oxidation state and the uptake or loss of electrons (after Meyer 1994). b Soil acidification as a result of N2 fixation: During N2 fixation, some plant roots release protons (derived from the dissociation of amino acids in the roots to keep ionic balance), which leads to acidification in many soil types. The low pH in the soil close to the pea root is in contrast to that close to the maize root, which raises the pH by absorbing nitrate. (Photo courtesy E. George). c, d Macrozamia communis as an example of the symbiosis between a plant and atmospheric N2-fixing bacteria. The cycads form on their hypocotyls so-called coralloid (or coral-like) roots from the cortex that contain cyanobacteria recognisable by the blue-green colouring of roots. Several species of Macrozamia grow in the nutrient-deficient forests of Australia. (Photo: E.-D. Schulze)
N2 fixation is low when N availability in the soil is high (e.g. in agriculture), because plants then use inorganic N forms instead of the energy-intensive N2, or when P availability to the plant is low, because of the high need for ATP (Eq. 16.19). One can see already from this first reaction in the N cycle that the oxidation number of N changes depending on the N compound in question (Fig. 16.23a). In addition to N2 fixation by bacteria, N2 can also be oxidised by lightning or fire (for further details, Sect. 21.2 in Chap. 21).


Autotrophic soil bacteria of the genera Nitrosomas (ammonification) and Nitrobacter (nitrification) are responsible for these reaction and use the energy released as energy sources. The real reactions are (even more) complicated (than shown), as for example during the formation of nitrite the oxygen used as electron acceptor comes not from O2 but from water. During the conversion of ammonium to nitrate, losses of intermediate products, particularly the gases NO2, NO and N2O, are also possible (Fig. 16.22). These losses affect the radiation balance of the Earth (Chap. 9).



Plants are able to take up different N species, NH4 + as well as NO3 − and small amino acids (Chap. 11). For more details on the consequences of N uptake for biodiversity, Sect. 20.4 in Chap. 20. Uptake can be below-ground via roots, but also above-ground. Here, atmospheric N deposition comes into play. Anthropogenic sources of reactive nitrogen include NOx, originating from burning and combustion processes, and NH3, mainly from livestock, fertilisation, sewage systems or industrial production of ammonium. The N deposition in ecosystems from such sources often exceeds 5 kg N ha−1 year−1 (Sect. 21.2, Chap. 21). In some areas with intensive industry or agriculture, N deposition can reach 20 to >50 kg N ha−1 year−1 (Bobbink et al. 2010). N deposition has been shown to be one of the main drivers for changing species composition in many ecosystems, mainly due to the outcomes of resource competition. In addition, N inputs into ecosystems trigger soil acidification and can (Sect. 16.4). Using stable nitrogen isotope labelling, the fate of N deposition can be followed. It turns out that plant biomass distribution within an ecosystem is not a good indicator of short-term N retention (Buchmann et al. 1996). Understorey vegetation was a larger N sink (9–15%) than 15-year-old Picea abies trees (3–7%), although tree biomass was a factor 4 larger than understorey biomass. The main N sink was the soil (79–87%), particularly the top soil (46–63%). A recent meta-study revealed that soil carbon stocks were a good predictor of 15N tracer retention in 48 ecosystems (Templer et al. 2012). Thus, soils represent a large sink for atmospheric N deposition, which can prevent N leaching as long as N deposition stays below approx. 46 kg N ha−1 year−1.
Plant annual N requirements can be met by N mineralisation, N2 fixation and N deposition or fertilisation. But also internal N fluxes, for example, remobilisation of amino acids prior to leaf shedding, can contribute to meeting these N requirements, although the extent varies widely. For example, N concentrations in litter vary between 5 and 10 mg g−1 dry matter in needles and leaves, up to 50 mg g−1 dry matter in crops and forage plants, and between 1 and 5 mg g−1 dry matter in wood. In a temperate beech forest, about 3 t dry matter ha−1 of litter reaches the soil each year, providing an annual input of plant-derived N of about 15–30 kg N ha−1 year−1 for litter decomposition and mineralisation and, thus, in turn for plant uptake. On the other hand, the N requirements of a temperate spruce forest in Germany could not be met by soil N availability alone: about 12% of annual N requirements were met by atmospheric N deposition via above-ground N uptake in the canopy (Horn et al. 1989), compared to 10% in Eastern US conifer sites (Sievering et al. 2000). Nave and Curtis (2011) estimated that about half of the N deposition is intercepted and taken up in forest canopies.
The N supply via N mineralisation (i.e. ammonification and nitrification) is highly variable with respect to both the chemical species and the amount. At very low supply (N deficiency) and in acidic soils, fungi are the dominant microorganisms in the soil (Smith and Read 2008). They can make organic nitrogen available via proteases, which are particularly active at low pH, that is, fungi acidify the substrate by releasing protons and thus directly take up organic nitrogen from litter. Fungi generally have a higher N requirement than plants since in several species the cell walls are formed by N-rich glucosamine (chitin). Fungi also require carbohydrates that they obtain as mycorrhizae either directly from plants or as saprophytes living on organic substances from the decomposition of litter and debris. Mycorrhizal fungisupply amino acids derived from protein degradation to the plant, in exchange for carbohydrates. In boreal coniferous forests, this “short-circuited” nitrogen cycle, bypassing mineralisation by microbes, is so effective that no free nitrate or ammonium may be found in the soil solution (Wallenda et al. 2000). Despite the dominance of fungi in the degradation of organic substances and in the uptake of N in boreal forests, there are also ammonium-forming and nitrifying bacteria, as shown by the presence of spores that become active after long incubation times (Persson et al. 2000). Ammonium and nitrate only occur in soil solutions in boreal climates when the supply of calcium is high, so the soil pH increases (Nordin et al. 2001). Bacteria are more effective than fungi at mineralizing organic matter at higher pH values. Under these conditions, nitrate and ammonium can be detected in the soil solution as the main N products of decomposition (Sect. 16.2). In soil, oxidation and reduction of nitrogen compounds can take place concurrently in all horizons, since the inside of soil aggregates provide oxygen-free (anaerobic) zones.
An excess of ammonium or nitrate, also termed nitrogen saturation, occurs when external supply (by N deposition, fertilisation) or formation in the ecosystem (by mineralisation) exceeds consumption (e.g. by seasonal variation of growth). Nitrate leaching of 5 kg N ha−1 year−1 is used as an indicator for N saturation thresholds. This has very significant consequences for the ecosystem (Sect. 16.4). Ammonium excess causes the release of cations, particularly K+ and Al3+ from clay minerals (Chap. 11). In contrast, the highly mobile nitrate ion is not bound to the soil exchanger. Thus, nitrate excess can lead to nitrate leaching to lower soil horizons, but also to groundwater and, further, drainage systems. This loss of anions is coupled to an equimolar loss of cations. Deposition of N from air pollutants often accelerates N transformations and leads to increased nitrate loss, even without interaction with organisms in the ecosystem (Durka et al. 1994; de Vries et al. 2003). As a result of high N deposition rates and negative environmental effects, the critical load concept was developed in the 1980s. It calculates a threshold of N deposition for ecosystems below which one does not expect any negative effects. The set critical load for most temperate forests is between 10 and 20 kg N ha−1 year−1. Over time, many studies have now identified the real critical loads. They range from 5 to 10 kg N ha−1 year−1 for boreal forests, tundra, bogs and alpine ecosystems to 20–30 kg N ha−1 year−1 for low- and medium-elevation hay meadows (Bobbink et al. 2010). The highest critical loads, 30–40 kg N ha−1 year−1, have been reported for salt marshes. Still, more than 25% of all European forests suffer under N deposition rates higher than their corresponding critical loads. However, long-term fertilisation trials in Swedish forests contradict the notion that high N deposition directly translates to N leaching. Despite N fertilisation rates between 20 and 100 kg N ha−1 year−1 (as ammonium nitrate, adding up to about 2000 kg N ha−1 over 30 years), no N leaching occurred; instead N additions were used for increased forest growth (Binkley and Högberg 2016). Magnani et al. (2007) found a very strong positive relationship between net ecosystem production (Sect. 16.2) and wet N deposition (<15 kg N ha−1 year−1) and questioned the risk of widespread N saturation under natural conditions. However, they were heavily criticised for their assumptions (de Vries et al. 2008).
16.4 Cation Fluxes in Terrestrial Ecosystems
Cation supply (in particular of K+, Mg2+, Ca2+) occurs mainly from chemical weathering of primary minerals (Chap. 11) or via dust particles and sea spray entering the ecosystem from the atmosphere. Examples of dust inputs are the formation of loess in the post-glacial period (Blume et al. 2010), the supply of dust from the Sahara to the Amazon delta (Worobiec et al. 2007) and buffering of sulphur-containing emissions by industrial dusts in the 1960s, which delayed acidification of soils, as the ionic charge of deposited material was neutral. The deposition of sea salts from sea spray in coastal areas, that is, sea salt aerosols formed from the ocean, results in strong gradients of Ca2+, Na+ and Mg2+, as well as Cl− and SO4 2− ions deposited on plant foliage to interior areas (Gustafsson and Franzen 2000).
Cations are taken up by roots and incorporated into plant tissues, where they remain for months to decades depending on foliage lifetimes and decomposition rates (Sect. 16.2.2). However, the return of cations into the soil occurs not only via decomposition but also through leaching from the canopy. Canopy leaching is a consequence of ammonium uptake from atmospheric pollution during which cations are leached buffering the input of protons. Thus, cation concentrations in throughfall are often larger than in bulk precipitation (Sect. 16.1). The accumulation of cations in the litter layer and in soil organic material and dense root systems in the top soil horizons enable direct resupply of cations from decomposing organic material to plants, particularly under nutrient-limited conditions (e.g. tropical and boreal systems). However, cations released from organic matter can also be leached from the soil together with DOC, for example, organic acids. As a consequence, cation concentrations (also aluminium and iron) in the soil solution of upper soil layers decrease and bleached horizons are formed, especially in nutrient-poor, sandy soils (eluvial E horizons). Alkaline saturation increases only in deeper soil layers (B and C horizons).
Cation balance in a spruce forest (in mmol m−2 year−1)
|
Calcium |
Potassium |
Magnesium |
|
|---|---|---|---|
|
Soil |
|||
|
Atmospheric input |
25 |
19 |
10 |
|
Leaching from canopy |
10 |
25 |
1 |
|
Weathering |
79 |
52 |
36 |
|
Release from litter |
189 |
54 |
29 |
|
Leaching into groundwater |
−45 |
−21 |
−33 |
|
Available in soil |
258 |
129 |
43 |
|
Plants |
|||
|
Incorporation into wood |
−59 |
−50 |
−13 |
|
Leaching from canopy |
−10 |
−25 |
−1 |
|
Litter |
−189 |
−54 |
−29 |
|
Total plant uptake |
−258 |
−129 |
−43 |

Schematic representation of nitrate and cation fluxes downslope resulting from groundwater run-off in a boreal forest in Scandinavia. Two different mechanisms could explain the high productivity of trees downslope. a High nitrate leaching and associated cation loss that accumulate downslope and increase resource availability for growth downslope. b Low nitrate loss but large cation loss in association with DOC. (After Högberg 2001)

a–d Changes in K, Mg, Al, Ca, P, and N concentrations in soils derived from weathered lava and in leaves along an age gradient on Hawaii. e, f The relative changes in soil nutrients compared to lava and g carbon pools as well as h NPP are also shown. (Vitousek et al. 1997; Chadwick et al. 1999)
Natural processes causing cation loss may be accelerated by strong acids, particularly if they form acid anions that then enter the groundwater. Sulphate, but also chloride and nitrate, belongs to these acid anions, insofar as they are not used in lower soil layers and, thus, enter the groundwater. Such strong acids do not occur in excess under natural conditions. However, since industrialisation, ecosystems have been increasingly impacted by the atmospheric deposition of acids. In Europe, this has caused soil acidification over a period of about 30 years (Schulze 1989), with base saturationin all soil horizons decreasing from 10 to 50% at the start of the period to 5% on average a few decades later (Ulrich 1987). In Sweden, some soils even lost 70% of their exchangeable base cations in the mineral soil, but not in the organic layer (Högberg et al. 2006).
-
The observed loss of needles and discolouration were natural and would have occurred without the atmospheric input of acids.
-
The damage was triggered by organisms (pests and pathogens).
-
The damage was a direct response to atmospheric pollutants or a consequence of acidification of soils.

Conceptual framework of a combination of factors leading to forest decline (Schulze and Lange 1990)
-
The primary impact of pests and pathogens could not be demonstrated, although several insects and pathogens (bark beetles, stem rot) attack weakened trees and, ultimately, cause their death.
-
During the twentieth century, high concentrations of pollutant gases, for example, SO2 and NOx, in emissions from industries caused damage, but the more recent damage symptoms were different. These new symptoms were related to tropospheric ozone, but the effect of ozone was shown to be rather complex and not straightforward (Chap. 5).
-
Acidification of soils causes root damage, particularly due to the release of mono-nuclear aluminium (Al) species at low pH values (increased solubilisation of Al oxides and Al silicates at pH < 5; Sect. 11.5 in Chap. 11). However, individual tree species react differently to Al. Furthermore, Al also reacts with phosphate in the mycorrhiza and is immobilised (Kottke and Oberwinkler 1986). Thus, soil acidification alone could not explain the observed damage.
-
One type of forest damage, characterised by yellowing of needles caused by magnesium deficiency, could be explained as a consequence of pollutants at the ecosystem scale (Schulze 1989). Soil acidification strongly reduces the availability of magnesium (and calcium) to plants. This is not only caused by the reduced base saturation of the soil exchanger occurring simultaneously with acidification, but also by competitive inhibition of Mg uptake by ammonium. In addition to ammonification, ammonium in the soil originates from atmospheric deposition, particularly from animal husbandry. Ammonium causes the release of Mg from exchangers in the soil and stimulates release of Al. Finally, Lange et al. (1989) proved in a very elegant experiment that the interaction of N with growth causes Mg deficiency in spruce. Buds were removed or not removed on opposite lateral twigs along the same branch. Damage was observed only on twigs where buds had not been removed and where growth had occurred (Chap. 11, Box 11.4). Obviously, the growth of trees is significantly regulated by N supply. Thus, canopy N uptake from airborne pollutants (in rain, fog and dew) becomes particularly important because this additional N supply is not balanced by cation uptake but leads to increased growth and the observed yellowing. The model of a nitrogen–cation interaction with limited cation supply due to soil acidification observed for forest decline could also be related to other observed cation deficiencies, particularly K deficiency on bogs, Mn and Fe deficiency on limestone, and the rarer Ca deficiency.

Effects of management on forests. Logging in the 1980s left free-standing single trees. With smaller S, but still high N deposition rates, the remaining individual trees grew faster. Despite fewer trunks and a smaller leaf area, the volume of growth per stand remained the same. (Data from Mund et al. 2002)
16.5 Summary
Water Fluxes
-
Ecosystems have no water cycle but rather a hydrological balance between precipitation (rain, snow, fog, dew, rime and hail) and evapotranspiration, run-off and seepage. About 60% of total terrestrial precipitation is returned to the atmosphere via evapotranspiration.
-
Ecosystem evapotranspiration, also called latent heat flux, is controlled by available energy and by plant ecophysiology. The Penman-Monteith equation describes this close link between energy and water fluxes.
-
Ecosystem evapotranspiration consists of canopy transpiration and soil evaporation. Big-leaf or two-leaf models are often used to scale up leaf transpiration to the canopy scale. Evergreen forests have higher evapotranspiration rates than deciduous forests; grasslands lose more water than forests when well supplied with water.
-
Canopy transpiration is controlled by two major drivers: leaf physiology (driving imposed evaporation) and available energy (driving equilibrium evaporation). Both evaporation processes occur at the same time and can be partitioned using the decoupling factor. Decoupling decreases with increasing height of vegetation and with smaller foliage of vegetation.
-
Forest and grassland ecosystems respond differently to drought, with forests increasing their ratio of gross primary production to evapotranspiration, thus cooling the atmosphere less than grasslands early in the drought. This changes when the drought prevails and the grassland vegetation wilts, thereby decreasing evapotranspiration and the cooling feedback to the atmosphere.
Carbon Fluxes
-
Important CO2 fluxes in terrestrial ecosystems are GPP and autotrophic and heterotrophic respiration (R e). About 50% of GPP is used for NPP. NPP is not the same as standing biomass or annual yield.
-
Soil respiration accounts for 50 to 70% of R e and is primarily driven by canopy assimilation. Environmental factors like temperature and moisture also have an effect. The decomposition of organic material comprises the decay of plant materials by soil fauna and microorganisms and mineralisation to inorganic forms, such as CO2 and CH4. Stabilisation of organic matter and decomposition products in the soil contribute to the long-term ecosystem carbon budget. Further C losses from ecosystems occur via leaching of dissolved carbon, harvests, fires and erosion, but also as BVOCs.
-
The NEP, determined by the difference between GPP and R e, is equivalent to the ecosystem carbon source or sink, when no further C inputs or exports occur. It can be measured with micrometeorological techniques. However, if the ecosystem is managed or disturbed, additional inputs and exports need to be considered, and the NBP represents the carbon sink or source strength.
-
Stable carbon isotopes and radiocarbon analyses can be used to determine the fate of carbon in an ecosystem and MRTs. The MRTs determine how fast organic materials are mineralised and returned to the atmosphere as CO2. MRTs are short for foliage and fine roots, longer for wood, and very long for SOM.
Nitrogen Fluxes
-
The transformations of nitrogen in terrestrial ecosystems represent almost a closed cycle. Many organisms of different trophic levels are involved.
-
Nitrogen enters an ecosystem via N2 fixation, by symbiotic and by free-living N2-fixing organisms, but also by lightning. Denitrification closes the ecosystem N cycle. Further inputs can be via fertilisation and N deposition, and further outputs occur via harvests, fire and leaching.
-
The conversion of different N species, for example, of ammonium into nitrate (ammonification) or of nitrate into ammonium (nitrification), is controlled by microbial processes that are dependent on the redox potential. Soil chemical conditions determine whether organic nitrogen, ammonium or nitrate is the dominant form in the N cycle. Anaerobic conditions lead to the conversion of nitrate to ammonia or to denitrification, which releases NO2, NO and N2O. These intermediaries are or can be transformed into greenhouse gases that affect climate.
-
Excess inputs of N into ecosystems can lead to N saturation, and leaching can occur, mainly as nitrate. The leaching of nitrate (and other strong acids) also results in equivalent cation losses, which are highly detrimental to ecosystem health.
-
Stable isotope tracer studies help to understand the fate of N in ecosystems. Soils are the largest sink for N deposition.
-
The concept of critical loads defines thresholds for N deposition into ecosystems below which no negative impacts are expected. They range from 5 to 40 kg N ha−1 year−1.
Cation Fluxes
-
Cation fluxes within ecosystems do not represent a closed cycle. Cations enter ecosystems naturally via weathering, dust or sea spray. Nowadays, atmospheric deposition plays an important role. Cycling within ecosystems includes uptake, release via decomposition and leaching from the canopy owing to atmospheric deposition and from the ecosystem because of podzolisation and soil acidification.
-
Leaching from the soil occurs with DOC and strong acids that are produced anthropogenically. These acids displace cations from the exchange sites and lead to soil acidification when the losses are not compensated by weathering of primary minerals, import of dust, or by liming operations.
-
Ecosystem flux rates of Ca2+ are larger than those of K+, and both exceed those of Mg2+.
-
Lateral transport of cations, together with the transport of DOC, can explain the differentiation of soil chemical characteristics and of vegetation even along short hydrological gradients.
-
Forest damage and decline and tree death are rarely due to one factor only, but most often to multifactorial combinations of stressors, including soil acidification, N-triggered growth and ozone, together with interactions of insect pests and microbial pathogens.