2.1 Preparation of Reagents of Specified Concentrations
Virtually every analytical method involving wet chemistry starts with preparing reagent solutions. This usually involves dissolving solids in a liquid or diluting from stock solutions. The concentration of analytes in solution can be expressed in weight (kg, g, or lower submultiples) or in the amount of substance (mol), per a unit volume (interchangeably L or dm3, mL or cm3, and lower submultiples). Preparing reagents of correct concentrations is crucial for the validity and reproducibility of any analytical method. Below is a sample calculation to prepare a calcium chloride reagent of a particular concentration.
Example A1
How much calcium chloride do you need to weigh out to get 2 L of a 4 mM solution?
Solution
![$$ M\left(\frac{\mathrm{mol}}{\mathrm{L}}\right) = \frac{n\ \left(\mathrm{mol}\right)}{v\left[\mathrm{L}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ1.png)




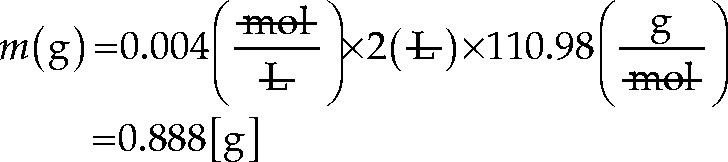
Example A2
How much would you need to weigh out if you were using the dihydrate (i.e., CaCl2 .2H2O) to prepare 2 L of a 4 mM solution of calcium chloride?
Solution

Thus, 1.176 g of CaCl2 would be weighed out and the volume made up to 2 L in a volumetric flask.
Concentration expression terms
|
Unit |
Symbol |
Definition |
Relationship |
|---|---|---|---|
|
Molarity |
M |
Number of moles of solute per liter of solution |
|
|
Normality |
N |
Number of equivalents of solute per liter of solution |
|
|
Percent by weight (parts per hundred) |
wt % |
Ratio of weight of solute to weight of solute plus weight of solvent × 100 |
|
|
wt/vol % |
Ratio of weight of solute to total volume × 100 |
|
|
|
Percent by volume |
vol % |
Ratio of volume of solute to total volume × 100 |
|
|
Parts per million |
ppm |
Ratio of solute (wt or vol) to total weight or volume × 1,000,000 |
|
|
Parts per billion |
ppb |
Ratio of solute (wt or vol) to total weight or volume × 1,000,000,000 |
|
Example A3
Prepare 500 mL of a 6 M sulfuric acid solution from concentrated sulfuric acid. The molecular weight is 98.08 g/mol, and the manufacturer states that it is 98 % wt/wt and has a density (d) of 1.84 g/mL.
Solution




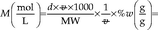

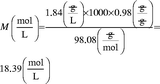
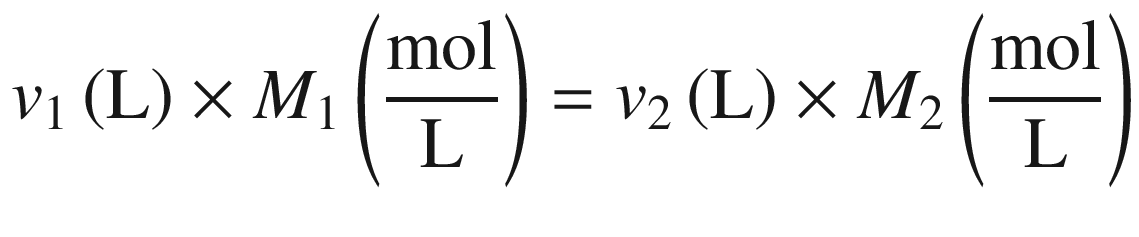


Hence, to obtain 500 mL, 163 mL concentrated sulfuric acid would be combined with 337 mL of water (500–163 mL). The dissolution of concentrated sulfuric acid in water is an exothermic process, which may cause splattering, and glassware can get very hot (most plastic containers are not suited for this purpose!). The recommended procedure would be to add some water to a 500 mL volumetric glass flask, e.g., ca. 250 mL, then add the concentrated acid, allow the mixture to cool down, mix, and bring up to volume with water.
2.2 Use of Titration to Determine Concentration of Analytes
-
A reagent of known concentration (i.e., the titrant)is titrated into a solution of analyte with unknown concentration. The used up volume of the reagent solution is measured.
-
The ensuing reaction converts the reagent and analyte into products.
-
When all of the analyte is used up, there is a measurable change in the system, e.g., in color or pH.
-
The concentration of the titrant is known; thus the amount of converted reactant can be calculated. The stoichiometry of the reaction allows for the calculation of the concentration of the analyte, for example, in the case of iodine values, the absorbed grams of iodine per 100 g of sample; in the case of peroxide value, milliequivalents of peroxide per kg of sample; or in the case of titratable acidity, % wt/vol acidity.



 = 49.04 g/mol, whereas for NaOH it
would be equal to the molecular weight since there is only one OH
group.
= 49.04 g/mol, whereas for NaOH it
would be equal to the molecular weight since there is only one OH
group.
Example B1
100 mL of vinegar is titrated with a solution of NaOH that is exactly 1 M. (Chapter 21, Sect. 21.2, in this laboratory manual describes how to standardize titrants.) If 18 mL of NaOH are used up, what is the corresponding acetic acid concentration in vinegar?
Solution

Example B2
Assume that 100 mL of apple juice are titrated with 1 M NaOH, and the volume used is 36 mL. What is the molarity of malic acid in the apple juice?
Solution

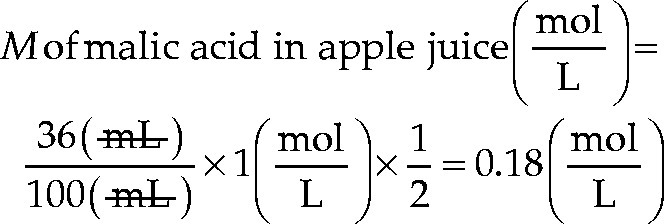
Tables, calculators, and other tools for calculating molarities, normalities, and % acidity are available in print and online literature. However, it is important for a scientist to know the stoichiometry of the reactions involved, and the reactivity of reaction partners to correctly interpret these tables.
The concept of normality also applies to redox reactions; only electrons instead of protons are transferred. For instance, potassium dichromate, K2Cr2O7, can supply six electrons, and therefore the normality of a solution would be six times its molarity. While the use of the term normality is not encouraged by the IUPAC, the concept is ubiquitous in food analysis because it can simplify and speed up calculations.
For a practical application of how normality is used to calculate results of titration experiments, see Chap. 21 in this laboratory manual.
2.3 Preparation of Buffers
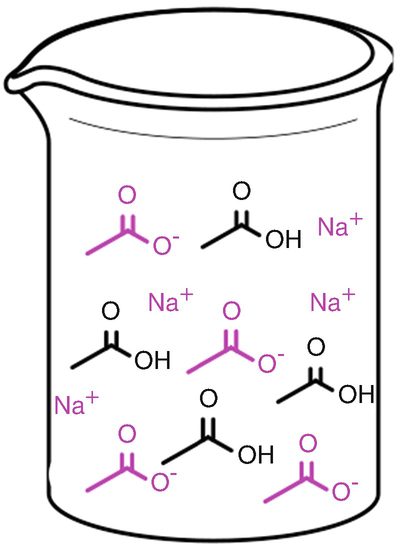
A buffer solution composed of the weak acetic acid, CH3COOH, and a salt of its corresponding base, sodium acetate, CH3COO−Na+. In aqueous solution, the sodium acetate dissociates into CH3COO− (acetate ions) and Na+ (sodium ions), and for this reason, these ions are drawn spatially separated. The Na+ ions do not participate in buffering actions and can be ignored for future considerations. Note that the ratio of acetic acid and acetate is equal in our example. Typical buffer systems have concentrations between 1 and 100 mM
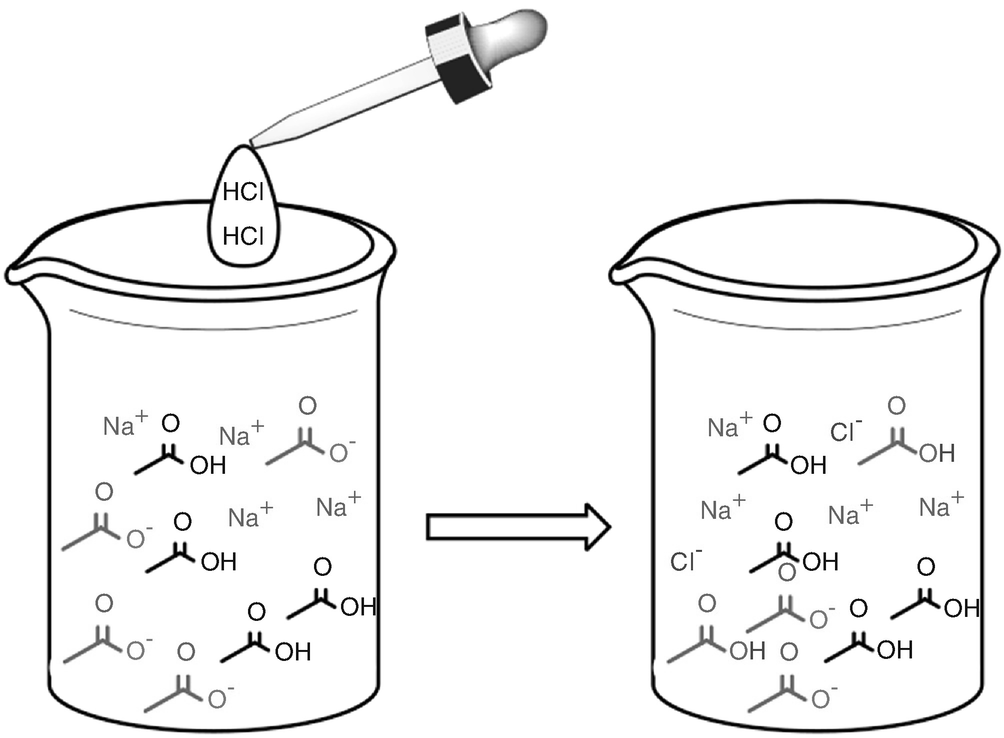
Changes induced by addition of the strong acid HCl, to the buffer from Fig. 2.1. HCl can be considered as completely dissociated into Cl− and H+. The H+ combines with CH3COO− instead of H2O, because CH3COO− is the stronger base. Thus, instead of H3O+, additional CH3COOH is formed. This alters the ratio between CH3COOH and CH3COO−, resulting in a different pH as illustrated in problem C2. The Cl− ions are merely counterions to balance charges but do not participate in buffer reactions and can thus be ignored
Properties of common food acids
|
Formula |
pKa1 |
pKa2 |
pKa3 |
Molecular weight |
Equivalent weight |
|
|---|---|---|---|---|---|---|
|
Acetic acid |
CH3COOH |
4.75b |
– |
– |
60.06 |
60.06 |
|
Carbonic acid |
H2CO3 |
6.4b |
10.3b |
– |
62.03 |
31.02 |
|
6.1c |
9.9c |
|||||
|
Citric acid |
HOOCCH2C(COOH)OHCH2COOH |
3.13b |
4.76b |
6.40b |
192.12 |
64.04 |
|
2.90c |
4.35c |
5.70c |
||||
|
Formic acid |
HCOOH |
3.75b |
– |
– |
46.03 |
46.03 |
|
Lactic acid |
CH3CH(OH)COOH |
3.86 |
– |
– |
90.08 |
90.08 |
|
Malic acid |
HOOCCH2CHOHCOOH |
3.5b |
5.05b |
– |
134.09 |
67.05 |
|
3.24c |
4.68c |
|||||
|
Oxalic acid |
HOOCCOOH |
1.25b |
4.27b |
– |
90.94 |
45.02 |
|
1.2c |
3.80c |
|||||
|
Phosphoric acid |
H3PO4 |
2.15b |
7.20b |
12.38b |
98.00 |
32.67 |
|
1.92c |
6.71c |
11.52c |
||||
|
Potassium acid phthalate |
HOOCC6H4COO−K+ |
5.41b |
– |
– |
204.22 |
204.22 |
|
Tartaric acida |
HOOCCH(OH)CH(OH)COOH |
3.03 |
4.45 |
– |
150.09 |
75.05 |
![$$ \mathrm{pH} = {\mathrm{pK}}_{\mathrm{a}} + \log\ \frac{\left[{\mathrm{A}}^{-}\right]}{\left[\mathrm{AH}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ25.png)
![$$ \mathrm{pOH} = {\mathrm{pK}}_{\mathrm{b}} + \log\ \frac{\left[{\mathrm{BH}}^{+}\right]}{\left[\mathrm{B}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ26.png)
Below are several examples of buffer preparation using the Henderson-Hasselbalch equation:
Example C1
What is the pH of a buffer obtained by mixing 36 mL of a 0.2 M Na2HPO4 solution and 14 mL of a 0.2 M NaH2PO4 solution, after adding water to bringing the volume to 100 mL to obtain a 0.1 M buffer?
Solution




Example C2
How would the pH of the buffer in Example C1 change upon addition of 1 mL of 2 M HCl? Note: You may ignore the slight change in volume caused by the HCl addition.
Solution






![$$ \mathrm{pH} = 6.71 + \log\ \frac{\left[0.052\right]}{\left[0.048\right]} = 6.74 $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ38.png)
Example C3
Prepare 250 mL of 0.1 M acetate buffer with pH 5. The pKa of acetic acid is 4.76 (see Table 2.2). The molecular weights of acetic acid and sodium acetate are 60.06 and 82.03 g/mol, respectively.
Solution
![$$ \mathrm{Molarity}\ \mathrm{of}\ \mathrm{buffer}\ \left(\frac{\mathrm{mol}}{\mathrm{L}}\right) = \left[\mathrm{AH}\right] + \left[{\mathrm{A}}^{-}\right] $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ39.png)
![$$ 0.1 = \left[{\mathrm{A}}^{-}\right] + \left[\mathrm{AH}\right] $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ40.png)
![$$ {[\mathrm{A}}^{\hbox{--} }] = 0.1\hbox{--} \left[\mathrm{A}\mathrm{H}\right] $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ41.png)
![$$ 5 = 4.76 + \log\ \frac{0.1-\left[\mathrm{AH}\right]}{\left[\mathrm{AH}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ42.png)
![$$ 0.24 = \log\ \frac{0.1-\left[\mathrm{AH}\right]}{\left[\mathrm{AH}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ43.png)
![$$ 1.7378 = \frac{0.1-\left[\mathrm{AH}\right]}{\left[\mathrm{AH}\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ44.png)
![$$ 1.7378 \times \left[\mathrm{AH}\right] = 0.1-\left[\mathrm{AH}\right] $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ45.png)
![$$ 1.7378 \times \left[\mathrm{AH}\right] + \left[\mathrm{AH}\right] = 0.1 $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ46.png)
![$$ \left[\mathrm{AH}\right] \times \left(1.7378 + 1\right) = 0.1 $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ47.png)
![$$ \left[\mathrm{AH}\right] = \frac{0.1}{1.7378+1} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ48.png)
![$$ \left[\mathrm{AH}\right] = 0.0365\ \left(\frac{\mathrm{mol}}{\mathrm{L}}\right) $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ49.png)
![$$ \left[{\mathrm{A}}^{-}\right] = \kern0.5em 0.1 - 0.0365 = 0.0635\ \left(\frac{\mathrm{mol}}{\mathrm{L}}\right) $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ50.png)
- 1.
Prepare 0.1 M acetic acid and 0.1 M sodium acetate solutions. For our example, 1 L will be prepared. Sodium acetate is a solid and can be weighed out directly using Eq. 2.5. For acetic acid, it is easier to pipet the necessary amount. Rearrange Eq. 2.8 to calculate the volume of concentrated acetic acid (density = 1.05) needed to prepare a volume of 1 L. Then express the mass through Eq. 2.5.
 (2.5)
(2.5) (2.50)
(2.50) (2.51)
(2.51) (2.52)
(2.52) (2.53)Dissolving each of these amounts of sodium acetate and acetic acid in 1 L of water gives two 1 L stock solutions with a concentration of 0.1 M. To calculate how to mix the stock solutions, use their concentrations in the buffer, i.e., 0.0365 as obtained from Eqs. 2.48 and 2.49 – 0.0365
(2.53)Dissolving each of these amounts of sodium acetate and acetic acid in 1 L of water gives two 1 L stock solutions with a concentration of 0.1 M. To calculate how to mix the stock solutions, use their concentrations in the buffer, i.e., 0.0365 as obtained from Eqs. 2.48 and 2.49 – 0.0365 for acetic acid and 0.0635
for acetic acid and 0.0635
 for sodium acetate and Eq.
2.15:
for sodium acetate and Eq.
2.15:
 (2.54)
(2.54) (2.55)
(2.55) (2.56)
(2.56)Combining the 0.091 L of acetic acid stock and 0.159 L of sodium acetate stock solution gives 0.25 L of buffer with the correct pH and molarity.
- 2.
Directly dissolve appropriate amounts of both components in the same container. Equations 2.48 and 2.49 yield the molarities of acetic acid and sodium acetate in a buffer, i.e., the moles per 1 liter. Just like for approach 1 above, use Eqs. 2.5 and 2.52 to calculate the m for sodium acetate and v for acetic acid, but this time use the buffer volume of 0.25 [L] to insert for v:

 (2.57)
(2.57) (2.58)
(2.58)Dissolve both reagents in the same glassware in 200 mL water, adjust the pH if necessary, and bring up to 250 mL after transferring into a volumetric flask.
Note: It does not matter in how much water you initially dissolve these compounds, but it should be > 50 % of the total volume. Up to a degree, buffers are independent of dilution; however, you want to ensure complete solubilization and leave some room to potentially adjust the pH.
- 3.
Pipet the amount of acetic acid necessary for obtaining 250 mL of a 0.1 M acetic acid solution, but dissolve in < 250 mL, e.g., 200 mL. Then add concentrated NaOH solution drop-wise until pH 5 is reached, and make up the volume to 250 mL. The amount of acetic acid is found analogously to Eq. 2.52:
 (2.59)
(2.59)
You will find all three approaches described above if you search for buffer recipes online and in published methods. For instance, AOAC Method 991.43 for total dietary fiber involves approach 3. It requires dissolving 19.52 g of 2-(N-morpholino)ethanesulfonic acid (MES) and 12.2 g of 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) in 1.7 L water, adjusting the pH to 8.2 with 6 M NaOH, and then making up the volume to 2 L.
However, the most common approach for preparing buffers is approach 1. It has the advantage that once stock solutions are prepared, they can be mixed in different ratios to obtain a range of pH values, depending on the experiment. One disadvantage is that if the pH needs to be adjusted, then either some acid or some base needs to be added, which slightly alters the volume and thus the concentrations. This problem can be solved by preparing stock solutions of higher concentrations and adding water to the correct volume, like in Example C1, for which 36 mL and 14 mL of 0.2 M stock solutions were combined and brought to a volume of 100 mL to give a 0.1 M buffer. This way, one also corrects for potential volume contraction effects that may occur when mixing solutions. Another potential disadvantage of stock solutions is that they are often not stable for a long time (see Notes below).
2.4 Notes on Buffers
- 1.
The buffer components need to be well soluble in water. Some compounds require addition of acids or bases to fully dissolve.
- 2.
Buffer recipes may include salts that do not participate in the buffering process, such as sodium chloride for phosphate-buffered saline. However, the addition of such salts changes the ionic strength and affects the acid’s pKa. Therefore, combine all buffer components before adjusting the pH.
- 3.
If a buffer is to be used at a temperature other than room temperature, heat or cool it to this intended temperature before adjusting the pH. Some buffer systems are more affected than others, but it is always advisable to check. For instance, 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid [HEPES] is a widely used buffer component for cell culture experiments. At 20 °C, its pKa is 7.55, but its change in pH from 20 to 37 °C is −0.014 ΔpH/°C [3]. Thus, the pKa at 37 °C would be:
 (2.60)
(2.60) (2.61)
(2.61) - 4.
Do not bring the buffer up to volume before adjusting the pH. Use relatively concentrated acids and bases for this purpose, so that the volumes needed for pH adjustment are small.
- 5.
Ensure that buffer components do not interact with the test system. This is especially important when performing experiments on living systems, such as cell cultures, but even in vitro systems are affected, particularly when enzymes are used. For instance, phosphate buffers tend to precipitate with calcium salts or affect enzyme functionality. For this reason, a range of zwitterionic buffers with sulfonic acid and amine groups has been developed for use at physiologically relevant pH values.
- 6.
Appropriate ranges for pH and molarities of buffer systems that can be described through the Henderson-Hasselbalch equation are roughly 3–11 and 0.001–0.1 M, respectively.
- 7.
The calculations and theoretical background described in this chapter apply to aqueous systems. Consult appropriate literature if you wish to prepare a buffer in an organic solvent or water/organic solvent mixtures.
- 8.
If you store non-autoclaved buffer or salt solutions, be aware that over time microbial growth or precipitation may occur. Visually inspect the solutions before use, and discard if cloudy or discolored. If autoclaving is an option, check if the buffer components are suitable for this process.
- 9.
When developing a standard operating procedure for a method that includes a buffer, include all relevant details (e.g., reagent purity) and calculations. This allows tracing mishaps back to an inappropriate buffer.
- 10.
There are numerous online tools that can help with preparing buffer recipes. They can save time and potentially allow you to verify calculations. However, they are no substitute for knowing the theory and details about the studied system.
2.5 Practice Problems
- 1.
(a) How would you prepare 500 mL of 0.1 M NaH2PO4 starting with the solid salt?
(b) When you look for NaH2PO4 in your lab, you find a jar with NaH2PO4 . 2H2O. Can you use this chemical instead, and if so, how much do you need to weigh out?
- 2.
How many g of dry NaOH pellets (molecular weight: 40 g/mol) would you weigh out for 150 mL of 10 % wt/vol sodium hydroxide?
- 3.
What is the normality of a 40 % wt/vol sodium hydroxide solution?
- 4.
How many mL of 10 N NaOH would be required to neutralize 200 mL of 2 M H2SO4?
- 5.
How would you prepare 250 mL of 2 N HCl starting with concentrated HCl? The supplier states that its concentration is 37 % wt/wt, and the density is 1.2.
- 6.
How would you prepare 1 L of 0.04 M acetic acid starting with concentrated acetic acid (density = 1.05)? The manufacturer states that the concentrated acetic acid is >99.8 %, so you may assume that it is 100 % pure.
- 7.
Is a 1 % wt/vol acetic acid solution the same as a 0.1 M solution? Show calculations.
- 8.
Is a 10 % wt/vol NaOH solution the same as a 1 N solution? Show calculations.
- 9.
What would the molarity and normality of a solution of 0.2 g potassium dichromate (molecular weight = 294.185) in 100 mL water be? It acts as an oxidizer that can transfer six electrons per reaction.
- 10.
How would you make 100 mL of a 0.1 N KHP solution? Note: For this practice problem, you only need to calculate the amount to weigh out, not explain how to standardize it. Chapter 21 of this laboratory manual provides further information about standardization of acids and bases.
- 11.
You want to prepare a standard for atomic absorption spectroscopy measurements containing 1000 ppm Ca. How much CaCl2 do you need to weigh out for 1000 mL stock solution? The atomic mass unit of Ca is 40.078, and the molecular weight of CaCl2 is 110.98 g/mol.
- 12.
Outline how you would prepare 250 mL of 0.1 M acetate buffer at pH 5.5 for enzymatic glucose analysis.
- 13.
Complexometric determination of calcium requires an ammonium buffer with 16.9 g ammonium chloride (molecular weight, 53.49) in 143 mL concentrated ammonium hydroxide solution (28 % wt/wt, density = 0.88; molecular weight = 17; pKb = 4.74). It is also to contain 1.179 g of Na2EDTA.2H2O (molecular weight = 372.24 g/mol) and 780 mg MgSO4 .7H2O (molecular weight = 246.47 g/mol).After combining all reagents, the volume is brought up to 250 mL. What are the molarities for EDTA and MgSO4, and what pH does this buffer have?
- 14.
Your lab uses 0.2 M stock solutions of NaH2PO4 and Na2HPO4 for buffer preparation.
- (a)
Calculate the amounts weighed out for preparing 0.5 L of fresh 0.2 M stock solutions of NaH2PO4 .H2O (molecular weight, 138) and Na2HPO4 .7H2O (molecular weight, 268).
- (b)
You want to make 200 mL of a 0.1 M buffer with pH 6.2 from these stock solutions. How many mL of each stock solution do you have to take?
- (c)
How would the pH change if 1 mL of a 6 M NaOH solution was added (note: you may ignore the volume change)?
- (a)
- 15.
Tris (2-amino-2-hydroxymethyl-propane-1,3-diol) is an amino compound suitable for preparation of buffers in physiological pH ranges such as for the dietary fiber assay; however, its pKa is highly affected by temperature. The reported pKa at 25 °C is 8.06 [3]. Assuming a decline in pKa of approximately 0.023 ΔpH/°C, what pH would a Tris buffer with a molar acid/base ratio of 4:1 have at 60 °C versus at 25 °C?
- 16.
Ammonium formate buffers are useful for LC-MS experiments. How would you prepare 1 L of a 0.01 M buffer of pH 3.5 with formic acid (pKa = 3.75, 98 % wt/wt, density = 1.2) and ammonium formate (molecular weight 63.06 g/mol)? Note: You may ignore the contribution of ammonium ions to the pH and focus on the ratio of formic acid/formate anion. You may treat the formic acid as pure for the calculation, i.e., ignore the % wt/wt.















![$$ \mathrm{pH} = 6.71 + \log\ \frac{\left[0.072\right]}{\left[0.028\right]} $$](/epubstore/N/S-S-Nielsen/Food-Analysis-Laboratory-Manual/OEBPS/images/104615_3_En_2_Chapter/104615_3_En_2_Chapter_TeX_Equ30.png)
